Abstract
Transplantation of insulin-secreting tissue represents a physiologic approach to reverse diabetes mellitus. Pancreas transplants yield a remarkable enhancement in quality of life and appear to modify the devastating neurovascular complications of diabetes. A more attractive approach is transplantation of insulin-secreting cells, a procedure of low invasiveness with the exciting prospect of modulating graft immunogenicity before transplantation, so as to minimize requirements for toxic immunosuppressive drugs. The Surgical-Medical Research Institute at the University of Alberta in Edmonton, and several others centres throughout the world, has demonstrated that islet cell transplants can reverse insulin dependence and induce remarkable glycemic stability for several years. However, widespread success has been denied because of insufficient donor tissue, early failures to reverse insulin dependence and the loss of graft function with time. Promising new research approaches to these problems are reviewed, including xenogeneic sources of cells, engineering islet cells with genes that induce expression of immunoprotective molecules, and neogenesis factors that may sustain populations of transplanted β cells.
Insulin-dependent diabetes mellitus remains a major health problem. Approximately 200 000 people require insulin therapy for diabetes in Canada, and the incidence is increasing annually. Diabetes is the fifth most common cause of death from chronic disease, and its devastating neurovascular complications, which may lead to stroke, gangrene, impotence, blindness, renal failure and cardiac disease, have a major impact on health care costs. Recently, data from the Diabetes Control and Complications Trial (DCCT) showed that intensive insulin therapy could significantly reduce the risk of neurovascular complications.1 This encouraging finding clearly sets the rationale for precise control of diabetes. However, the intense treatment used in the DCCT may not be generalizable to community practice and is associated with serious hypoglycemic episodes.
Transplantation of insulin-producing tissue represents a more attractive approach. This is because insulin-producing beta cells of the pancreas are restored, and they can respond to neural and humoral mediators to precisely regulate insulin delivery in response to daily needs. The only treatment for diabetes mellitus that normalizes circulating glucose throughout the day, resulting in normal glycosylated hemoglobin (HbA1c) without hypoglycemia is transplantation of the pancreas.2
Pancreas transplantation
Transplantation of the pancreas as a vascularized organ has become popular since 1966, with results of more than 8500 grafts being reported to the International Pancreas Transplant Registry by 1996.3 Analysis of recent data in pancreas transplantation reveals that 80% of grafts survive at 1 year. Patient survival exceeds 95% at 1 year and patient quality of life is remarkably enhanced through freedom from insulin therapy and dietary constraints. Most pancreas transplant procedures have been performed in conjunction with renal transplantation in patients suffering secondary complications of diabetes leading to end-stage diabetic nephropathy.
In spite of outstanding improvements in glycemic control, effects of pancreas transplantation on diabetes complications are not so clear. Multicentre, controlled trials to examine the effects of pancreas transplantation on the serious complications have not been completed. Individual reports suggest that peripheral but not autonomic neuropathy is improved. When accompanied by pancreas transplantation, the morphologic characteristics of simultaneously transplanted kidneys are improved, but kidney function has not been improved, presumably because of the immunosuppression needed for the transplantation. Retinopathy appears to be stabilized, but more prolonged periods of follow-up are needed to demonstrate definite benefits. Recent data suggest that long-term normoglycemia established by pancreas transplantation in nonuremic patients can reverse secondary complications.4 These encouraging results provide a strong rationale to continue pancreas transplantation, but long-term follow-up studies are still needed.
Balancing the remarkable advantages is the sobering morbidity associated with simultaneous kidney and pancreas transplantation. Compared with kidney transplantation alone, more bouts of infection, rejection, hospitalization and surgical complications can be expected.5 One study reported a 3-year patient survival rate of 68% compared with 90% for kidney transplantation alone.6 These results contrast with recent more favourable data in the International Pancreas Transplant Registry and highlight a need for careful selection criteria to offer combined pancreas and kidney transplants. A high incidence of malignancy has also been observed after combined pancreas and kidney transplantation. 7 These factors add up to significant costs to health care systems, which are struggling with limited resources.
Advances in surgical technique and reduced immunosuppressive drug-induced nephrotoxicity with tacrolimus promoted attempts at solitary pancreas transplantation for nonuremic patients with labile diabetes mellitus, recently the subject of a retrospective review.8 Patient survival at 1 year was 90%, pancreas survival at 1 year was 80% and serious surgical complications leading to repeat laparotomy were reduced to 8%. These preliminary data were observed in small numbers of recipients and graft function appeared to decrease sharply after 1 year.
It appears that pancreas transplantation offers remarkable improvement in quality of life for diabetic patients who have end-stage diabetic nephropathy requiring renal transplantation. However, this combined procedure is associated with significant morbidity and mortality, and the effect on the secondary complications of diabetes remains un-substantiated by scientifically rigorous trials. These factors have encouraged investigations with islet cell transplants.
Transplantation of isolated islets of Langerhans
Islet cell transplantation offers the potential of a low-level invasive procedure to the recipient. Whereas long-term immunosuppression is needed to prevent auto- and alloimmune destruction of pancreas grafts, experimental studies indicate that cell grafts can be immunomodulated to reduce the need for aggressive, toxic immunosuppression of recipients.9 An overview of some recent studies frames current challenges and controversies in this emerging field.
The University of Alberta experience in clinical islet transplantation
Six insulin-dependent diabetic patients aged 22 to 36 years who suffered from end-stage diabetic nephropathy received simultaneous kidney and islet cell transplants. Results were compared with a cohort of 9 patients who received a kidney transplant only.
In 2 patients, 4000 islets/kg body weight from one pancreas donor were embolized to the liver through a mesenteric vein during the kidney transplant procedure. In the other 4, supplements of islets, which were collected from 3 to 10 additional donors, were cryopreserved, then thawed and implanted, providing a total islet dose of 10 000 islets/kg body weight. In all instances, immunosuppression was with polyclonal antilymphocyte serum, cyclosporine, steroids and azathioprine. Prolonged follow-up has been maintained.
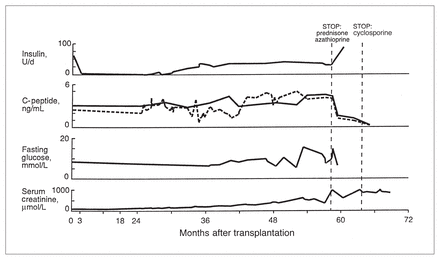
Patients who received 4000 islets/kg achieved insulin production (detected by C-peptide release from the grafts) of 1 to 2 ng/mL during fasting and 3 to 4 ng/mL after eating a mixed meal. This was sufficient to maintain glycemic control and reduce exogenous insulin requirements, but graft function decreased after 8 weeks. In the remaining 4 patients who received larger grafts of multidonor fresh plus cryopreserved islets, enhanced insulin secretion was observed. In one of these, function was sufficient to permit complete cessation of insulin therapy.10 Fig. 1 demonstrates the natural history of graft function in this patient. Insulin independence was maintained for over 2 years, during which time she had a normal HbA1c level. After 2 years, glycemic control deteriorated, necessitating a return to small daily doses of insulin therapy. Renal allograft function also deteriorated due to chronic renal rejection. Islet function persisted with detectable C-peptide secretion at 5-year follow-up. When immunosuppressive drugs were stopped, prompt loss of C-peptide secretion was observed, associated with increased insulin requirement.
Life and death of transplanted insulin-producing islets. Transplantation of fresh plus cryopreserved insulin-producing islet cells restored insulin secretion in this insulin dependent diabetic patient. She had sufficient insulin production to completely stop insulin injections for over 2 years after transplantation. This was accompanied by normal HbA1c level and normal glucose tolerance. After 2 years, graft function deteriorated resulting in a need for exogenous insulin supplements. Graft function persisted at 5-year follow-up. Rejection of a simultaneously implanted kidney led to cessation of immunosuppressive drugs which prompted loss of insulin secretion at 5-year follow-up. For C-peptide measurement, the dotted line = basal levels, the solid line = stimulated levels.
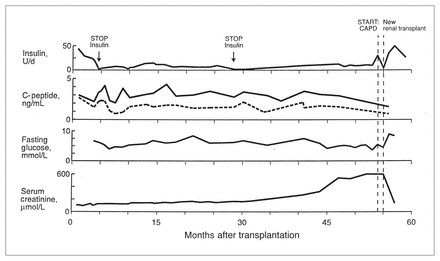
The other recipients of multidonor islets also demonstrated increased insulin secretion with more sustained function compared with the first 2 recipients of single donor islets. In these 3 subjects, C-peptide secretion has also persisted during prolonged follow-up. Fig. 2 demonstrates the natural history of graft function in a second patient who was insulin-independent for repeated intervals during 5 years. This patient also had a normal HbA1c level. Renal allograft rejection led to a need for repeat renal transplantation at 5 years and this was associated with deteriorated islet cell function.
Prolonged survival of transplanted islets. Second recipient of fresh and cryopreserved islets who had sustained islet cell graft function during a 5-year follow-up. Remarkable stability in glycemic control led to brief (1 to 3-week) intervals of insulin independence. However, chronic rejection of the simultaneously-implanted kidney led to a need for repeat renal transplantation, and this has been associated with deterioration in insulin secretion from the islet cells. CAPD = chronic ambulatory peritoneal dialysis.
A comparison with the renal-transplant-alone cohort yielded the following observations. In the cohort group, patient survival at 1 and 5 years’ follow-up was 85% and 56%, respectively. Deaths were attributable to sepsis (2 patients), myocardial infarction (1 patient), and cerebrovascular accident (1 patient). In the islet cell recipients, there was 1 death due to sepsis at 3 years, with 83% survival at 5 years. In the islet cell-kidney recipients, HbA1c levels remained within a normal range (less than 0.06) during the first and second year of follow-up. Those who received renal transplants alone, had poor glycemic control, with HbA1c levels consistently greater than 0.07 throughout the follow-up.
In summary, simultaneous kidney and intraportal islet cell transplantation at the University of Alberta restored insulin secretion in all 6 insulin-dependent diabetic patients with C-peptide levels greater than 1.0 ng/mL. Islet cell function was improved when combinations of multidonor fresh islet cells plus cryopreserved islet cells were implanted. No complications occurred as a result of the transplant procedures. Two patients completely stopped their insulin injections but returned to supplements of exogenous insulin after 2 years. The amount of insulin secreted from the graft decreased as the duration of follow-up increased.
The worldwide effort in islet cell transplantation
The International Islet Transplantation Registry in Giessen, Germany, has reported results of 294 grafts completed by multiple centres throughout the world to the end of December 1996.11 Eleven percent of the recipients were rendered insulin independent for periods of more than 1 week. At 1-year follow-up, 7% remained insulin independent. Detailed analysis of 96 patients who were initially C-peptide deficient before transplantation showed that detectable insulin secretion was lost in 60% during the first 2 months after transplantation. This function declined further so that only 27% had functioning grafts at 1-year follow-up (C-peptide greater than 1 ng/mL). Four common characteristics were identified among those with successful islet cell grafts: preservation of the pancreas for less than 8 hours before islet cell isolation, an islet cell dose greater than 6000/kg body weight, embolization of islet cells into the liver through the portal vein and use of antilymphocyte globulin to induce immunosuppression. When these criteria were fulfilled, 70% of patients had C-peptide levels greater than 1 ng/mL at 1 year follow-up and insulin independence was observed in 20%.
A striking result of the Registry data is that the graft environment heavily influences the outcome. Only 6 of 15 islet cell allografts after pancreatectomy-diabetes resulted in insulin independence at 1 year, but this was further reduced to 11 of 180 after allotransplantation into insulin-dependent diabetes. Thus, an allogenic environment is adverse, but in combination with autoimmune insulin-dependent diabetes, the success rate is even worse. Further, islet cells collected from patients subjected to total pancreatectomy for chronic pancreatitis then autografted into the recipient were associated with a 90% rate of insulin independence. This poses the following questions: Why do autografts work whereas allografts are less successful? and Why are allografts in insulin-dependent diabetes even less successful? Detailed examination of these questions provides several hypotheses. One is that immunosuppressive drugs are known to have diabetogenic side effects. Furthermore, islet cell grafts require a critical period of several days engraftment until a network of new capillaries nourishes the graft. Neo-vascularization may be impaired in long-standing diabetes. The microenvironment in which cells are injected is characterized by an inflammatory reaction with cytokines such as interleukin-1β, interferon-γ, and tumour necrosis factor-α. Insulin-producing β cells of the pancreas are extremely susceptible to reactive oxygen metabolites induced by such cytokines because they have low levels of antioxidants, which buffer these reactions. Nonspecific inflammatory mediators are greatly augmented in autoimmune diabetes, which may explain why function is rapidly lost in 60% of grafts. Clearly, islet cell transplants face a more difficult microenvironment than whole pancreas transplants.
A review of data in the Registry provokes pessimism over the future of islet cell transplantation. In contrast, observations presented here and previously, 12,13 show that islet cells can function for many years after implantation and contribute to remarkable stability of glycemic control. By the rigorous standards of pancreas transplantation, insulin independence is not achieved; however, the goal of optimizing precise glycemic control with minimal complications is consistent with DCCT data. Careful selection of recipients, immunosuppression, islet cell mass and perioperative care can achieve a direct benefit for the patient, and these observations need to be repeated with more studies. Rigorous comparisons of islet cell transplantation with intensive insulin therapy in well-designed prospective multicentre trials need to be completed. These trials should consider outcomes based upon glycemic control regardless of whether insulin independence is achieved.
Research progress towards effective islet cell transplantation
The key impediments to successful islet cell transplantation are: insufficient supply of donor tissue, autoimmune or alloimmune-mediated injury of transplanted islets, and deterioration of transplanted islet function with time. Progress has recently been made, providing novel insight into solutions to these problems.
The supply of donor tissue
To promote trials of transplantation for diabetes in Canada, a key hurdle to overcome is the short supply of donor pancreatic tissue. Statistics show a rate of cadaver kidney transplantation in Canada of 24 per million population per year. This amounts to about 360 pancreases expected per year. The Canadian Organ Replacement Register reported that 19 pancreases were transplanted in 1996. Another 30 were subjected to isolation of islet cells. The shortfall in utilization of donors is obvious when one estimates that 25% of kidney transplants are into insulin-dependent patients who have end-stage diabetes and that there are 200 000 diabetic patients taking insulin in Canada. Only when more pancreases are retrieved can research be promoted to perfect methods for the isolation and preservation of islet cells.
Recent attention has been attracted to xenografts of insulin-producing cells from pig donors as a solution to insufficient donor islets. Our laboratory has explored the preparation of mass quantities of islet cell clusters from the pancreas of neonatal piglets.14 In this model, growth and differentiation of cell clusters is observed in vitro. Following a maturation period the islet cells induced euglycemia after implantation into diabetic mouse recipients. This proliferating cell population opens new possibilities for expansion of transplantable cells in vitro. A major challenge is to limit the xenogenic destruction of such grafts using transgenic technologies or retroviral-mediated transfer of immunomodulatory genes.
Prevention of immune injury to transplanted islets
A key to successful islet cell replacement is to reduce or eliminate requirements for toxic immunosuppressive drugs. This would permit wide dissemination of islet cell transplantation for young insulin-dependent diabetics before serious secondary complications develop. We have recently investigated novel strategies to modulate the immunogenicity of islet cell tissue in vitro. Biolistics (a technique of bombarding tissue with microparticles of gold coated with DNA) was used to transfer immunomodulatory genes into intact adult islet cells. Biolistic-treated islet cells remained viable and restored euglycemia after transplantation into diabetic recipients. Biolistics was used to deliver genes encoding CTLA4Ig (a molecule that blocks the costimulatory molecule permitting T-cell activation) to islet cells. Transplantation of these islet cells reversed diabetes without immunosuppression in a significant number of recipients.15 This approach for in-vitro treatment may prove useful for islet cell protection using a variety of strategies, for example blocking T-cell activation, neutralizing proinflammatory cytokines in the graft microenvironment and inducing apoptosis in cytotoxic T cells. Such strategies may also prove useful to engineer cells to express insulin secretion characteristics and proximal signals that regulate insulin release.
Induction of long-term proliferation and survival of transplanted islet cells
As shown in the International Islet Transplantation Registry data and our own studies, islet cell function deteriorates with time. Emerging data on islet cell differentiation and proliferation in the adult pancreas offers room for optimism that this can be addressed. Proliferation can be induced by ilotropin, a protein cocktail prepared from the partially obstructed adult pancreas.16 Islet cell neogenesis gene-associated protein (INGAP) is a novel islet cell differentiation factor that confers bioactivity on ilotropin.17 This protein may be the essential link needed to rescue populations of transplanted β cells of the pancreas. A greater understanding of these factors may allow successful resolution of the problems with loss of islet function as the duration of follow-up increases after islet transplantation.
Conclusions
Impressive progress has been made in whole pancreas transplantation during the past 3 decades. Concerns persist that pancreas transplantation will not be a long-term answer for diabetes management, because of morbidity and mortality associated with the procedure and the lack of solid outcome data demonstrating a beneficial effect on secondary complications of diabetes. Islet cell transplantation provides an attractive alternative because of low invasiveness and reduced immunosuppression. Successful islet cell transplantation remains elusive. Recent data prove that some islet cell transplant recipients enjoy years of glycemic stability, and this should serve as a flashpoint for further investigations. Promotion of an adequate source of free islet cell grafts and protection of islets from molecular mediators of autoimmune or alloimmune injury deserve further emphasis. The goal of long-term islet cell function will be met when current research clarifies means to sustain survival of a transplanted β-cell population.
Acknowledgments
I gratefully acknowledge the assistance of Colleen Ruptash in preparation of this manuscript. Funding for University of Alberta studies presented in this manuscript was obtained from the Health Services Research Innovation Fund of the Alberta Heritage Foundation for Medical Research, Alberta Foundation for Diabetes Research and a Diabetes Interdisciplinary Program Grant from the Juvenile Diabetes Foundation International.
- Accepted August 26, 1998.