Abstract
Objective: To determine the prognostic value of flow cytometric analysis (S-phase fraction and DNA index) performed on lymph-node metastases of patients with stage III melanoma.
Design: A retrospective chart review with flow cytometric analysis of paraffin-embedded tissues.
Setting: A university teaching hospital.
Patients: Among 332 patients with cutaneous melanoma, 33 with stage III were identified. Distant metastases developed in 16 patients; 17 had no further recurrence. Charts were reviewed to obtain clinicopathologic parameters such as sex, age, location of the primary tumour, histologic features, presence or absence of ulceration, and Clark’s and Breslow’s levels.
Intervention: DNA ploidy and S-phase fraction were determined on the paraffin-embedded nodes.
Main outcome measures: The groups with or without recurrence were compared in terms of disease-free survival (DFS) and overall survival (OS). These survival parameters were correlated with DNA ploidy and S-phase fraction.
Results: By univariate analysis, clinicopathologic factors did not predict OS. A higher Clark’s level of invasion and more than 3 positive lymph nodes were associated with shorter DFS (p < 0.05). Tumour thickness and S-phase fraction did not correlate with either DFS or OS. Patients with diploid lymph-node metastases had an 87% 12-month survival compared with 41% for those with aneuploid tumours.
Conclusions: DNA ploidy may be used as a prognostic index in patients with lymph-node metastases. This could be particularly useful in the context of sentinel lymph-node mapping by which more patients are being identified with single microscopic lymph-node involvement.
The incidence of cutaneous malignant melanoma is increasing at a rate faster than any other cancer in United States and Canada. According to the American Cancer Society, approximately 7200 patients will die of metastatic melanoma over the next 10 years.1 The prognosis in patients with stages I and II cutaneous melanoma can be predicted using a number of morphologic variables including Breslow’s thickness, Clark’s level of invasion, the presence of ulceration or regression, host inflammatory cell response, growth pattern and blood vessel invasion. 2–8 These have been well characterized, and multivariate analysis has identified Breslow’s thickness as the single most important prognostic factor in primary cutaneous malignant melanoma. For stage III melanoma (TNM classification, regional nodal involvement), however, the number of prognostic factors is far less and includes only number and percentage of positive lymph nodes as well as the presence of extranodal extension.9–12 With the advent of sentinel lymph-node mapping, increasing numbers of patients are being identified with single microscopic nodal involvement, making prediction of the prognosis more difficult.13,14
To further classify patients into high- and low-risk groups, flow cytometry has been used primarily in stages I and II melanoma, with DNA ploidy emerging as an important prognostic factor.15–20 Little work has been done on stage III melanoma. A single study has demonstrated that DNA analysis of melanoma metastases with flow cytometry might provide additional prognostic information.21
Recent publications have shown benefits of interferon-alpha therapy in patients with high-risk resected cutaneous melanoma.21–23 The purpose of this study was to determine whether DNA ploidy and S-phase fraction of lymph-node metastases could improve prognostication in patients having stage III melanoma, thus providing a better guide to the use of adjuvant therapy.
Patients and methods
From 1986 to 1996, 332 patients with primary cutaneous melanoma were seen at the Royal Victoria Hospital in Montreal. Of these, 33 patients (21 men, 12 women) underwent a therapeutic lymph-node dissection with paraffin-embedded lymph-node specimens available for analysis. DNA analysis was carried out on all specimens, but determination of the S-phase fraction was possible on only 30 (91%). The median age of the 33 patients was 54.2 years. Medical charts were reviewed for clinicopathological information such as age, sex, anatomic location of the primary lesion, Breslow’s thickness, Clark’s level of invasion, presence of ulceration, number of positive lymph nodes and extranodal extension. Patient survival was determined as the time from nodal dissection to the date of last follow-up. Among these 33 patients, 5 had radiotherapy, 2 received immunotherapy and 1 had chemotherapy. Follow-up was completed at the time of data collection and all deaths were attributable to melanoma. The median follow-up was 29 months.
Flow cytometry
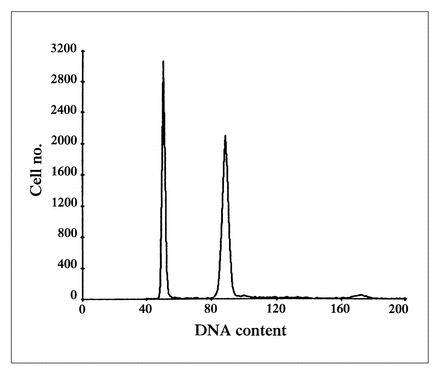
Tissue sections (50 μm thick) from representative formalin-fixed paraffin blocks of metastatic lymph nodes were taken. Specimens were dewaxed in xylene and rehydrated in a stepwise fashion. Enzymatic digestion was done using pepsin solution (0.5%; Sigma Chemical, St. Louis). After a wash in phosphate-buffered saline, the suspended nuclei were stained with propidium iodide and incubated in the dark at 4 °C overnight. The nuclear suspension was then filtered and submitted to flow cytometric analysis using an argon laser at 488 nm (Epics Profile II; Coulter Electronics, Hialeah, Fla.). Specimens were considered diploid if only one G0/G1 peak was present. Aneuploid histograms were defined as the presence of at least 2 separate peaks, each having at least 5% of the total number of events and each with a coefficient of variance of less than 5 (Fig. 1). The percentage of cells in the S-phase of the cell cycle was determined using the multicycle software with a nonlinear least square statistic and debris subtraction (Phoenix Flow System, San Diego).
DNA ploidy histogram of a melanoma metastatic to an inguinal lymph node. The first peak at channel 50 represents the diploid content of the normal lymphoid tissue, serving as an internal control. The second peak at channel 97 represents the aneuploid melanoma cells with a DNA index of 2. The small peak on the right of the histogram is the dividing population of the malignant cells in the G2/M phase of the cell cycle.
Statistical analysis
Survival analysis was performed using the Kaplan–Meier method. The Mantel–Cox statistical test was used for all DNA analyses and univariate analysis for the clinocopathological characteristics.
Results
The median (and standard deviation) disease-free survival (DFS) for the entire group was 10.3 (7.6) months, and median overall survival (OS) was 14.5 (6.3) months. The distribution and outcome of the 33 patients according to their clinicopathological characteristics are shown in Table I. Among the various factors analysed, only sex approached significance for predicting overall survival (p = 0.068). Clark’s level of invasion appeared to be a significant predictor for DFS (p = 0.03); however, there was only 1 patient in the level V group.
Survival and Clinicopathologic Features of 33 Patients Having Stage III Cutaneous Melanoma
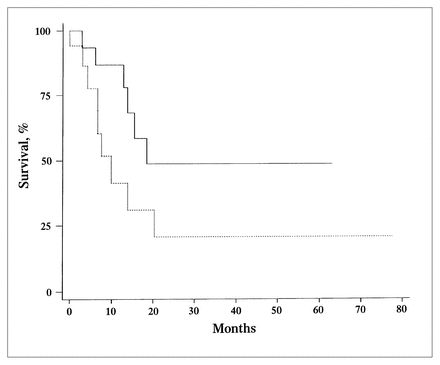
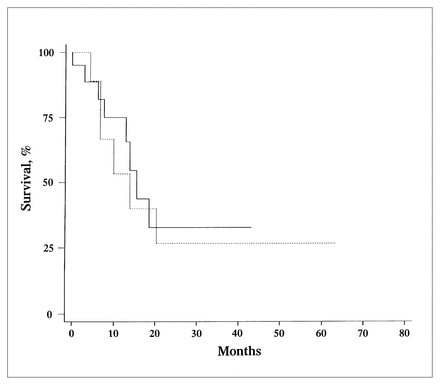
Analysis of lymph-node-related variables is summarized in Table II. Only DNA index (DI) correlated with outcome. The 12-month survival was only 41% for those with aneuploid lymph nodes (DI different from 1), compared with 87% for those with a DI equal to 1 (p = 0.06) (Fig. 2). An S-phase fraction lower than 6% was also associated with higher survival at 12 months (70% compared with 53% for an S-phase fraction 6% or greater). This difference, however, was not statistically significant (Fig. 3).
Kaplan–Meier analysis of 12-month survival of patients with stage III melanoma according to ploidy status. Patients with aneuploid lymph nodes had an index different from 1 (dotted line); those with diploid lymph nodes had an index equal to 1 (solid line) (p = 0.06).
Kaplan–Meier analysis of 12-month survival of patients with stage III melanoma according to low (less than 6, solid line) and high (6 or greater, dotted line) S-phase fractions (p = 0.71).
Survival and Flow Cytometry Results in 33 Patients Having Stage III Cutaneous Melanoma
Discussion
Although cutaneous malignant melanoma represents less than 2% of all cancers,1 its incidence has steadily increased over the last 2 decades. Primary or nonmetastatic melanoma has been studied extensively, with a number of clinicopathological parameters being identified as important predictors of prognosis. These include Breslow’s thickness, Clark’s level of invasion, presence or absence of ulceration and tumour location.2–8 Unfortunately, none can be used to predict prognosis of stage III patients. In fact, in this group, studies have shown that only the number of lymph nodes involved and presence of extracapsular extension are significant predictors of outcome.9–12
Two recent developments have generated a need to subdivide stage III patients into high- and low-risk groups. One is the advent of sentinel lymph-node mapping, which allows the clinician to identify an involved node without performing a complete node dissection with its attendant morbidity. Morton and colleagues24 have shown that when the sentinel lymph node is invaded, at least 78% of patients have only one lymph node involved in the entire node basin. Thus, we are beginning to identify a large cohort of patients with stage III disease in whom traditional prognostic factors are no longer useful. Second, Kirkwood and associates23 have recently demonstrated the efficacy of interferon alpha in stage III melanoma. As we begin using this agent clinically, it will become imperative that we develop novel prognostic markers to predict subsets of patients who will respond best. The purpose of our study was to determine whether DNA ploidy or S-phase fraction can be used to predict DFS and OS in patients with stage III melanoma.
S-phase fraction was found not to be related to OS or DFS. Hansson and colleagues25 (Table III16,17,19,20,22,25–30) found that the S-phase fraction was related to survival; however, they used 10% as their cut-off compared with the more stringent 6% used in this study. In fact only 2 of our patients had an S-phase fraction greater than 10%. Karlsson and associates,26 using paraffinembedded specimens, demonstrated that the S-phase fraction is a significant predictor of survival. In our study, the S-phase fraction was higher in those patients who died, but this difference did not reach statistical significance; therefore larger studies should be performed to obtain an adequate number of patients before reaching a conclusion about the usefulness of this parameter.
Review of the Literature on Melanoma and Flow Cytometry
In this study, DNA ploidy measured on metastatic lymph nodes was associated with improved survival with a probability value approaching statistical significance. Heaton and associates, 22 also looking at metastatic lymph nodes, found that DNA indices greater than 2 were significant predictors of poor patient survival. Hansson and colleagues25 found a similar correlation between prognosis and DNA index. Only Muhonen and colleagues,27 surprisingly, found that aneuploidy was associated with longer survival. This unusual association may be explained by the fact that most of their patients received systemic chemotherapy, and aneuploid tumours seem to have a greater response to chemotherapy than do diploid tumours. Most other published papers on DNA ploidy examined stages I and II disease, and the primary cutaneous lesions rather than the metastases as in our study.
With the advent of sentinel lymph-node mapping and the increasing use of interferon alpha we believe that accurate prognostic markers must be identified. This study has investigated one potential marker technique, flow cytometry. Results must be confirmed on a larger cohort of patients, but it appears that DNA ploidy may be useful for better stratification and further subset analysis of patients assigned to adjuvant therapy.
Footnotes
Presented in abstract form at the meeting of the American Association for Cancer Research, Washington, DC, Apr. 20 to 24, 1997.
- Accepted December 10, 1998.