Abstract
Objective: To assess factors affecting survival and pelvic recurrence after surgery and postoperative chemoradiation for rectal cancer in order to design improved management strategies.
Design: A chart review.
Setting: The British Columbia Cancer Agency.
Patients: One hundred and ninety-one consecutive patients who had rectal cancer treated between 1985 and 1994. Median follow-up was 39 months.
Interventions: Surgical excision of the cancer with intent to cure followed by chemoradiation.
Outcome measures: Multivariate analysis, to determine whether survival and pelvic recurrence were affected by tumour stage, nodal status, type of surgical procedure and presence of residual disease, and the quality of pathology reporting with respect to evaluation of radial resection margins and number of lymph nodes examined.
Results: Overall 5-year disease-specific survival was 60% and pelvic recurrence was 25%. Survival was affected by tumour stage (p < 0.02), nodal status (p < 0.001), type of surgical procedure (p < 0.04), presence of residual disease (p < 0.02) and pelvic recurrence (p < 0.0001). Pelvic recurrence was affected by the presence of residual disease (p < 0.001) but not by tumour stage (p < 0.14), nodal status (p < 0.37) or type of surgcial procedure (p < 0.20). Radial margins were evaluated in 44% of pathology reports and the median number of lymph nodes assessed was 6.
Conclusions: Survival was most significantly affected by pelvic recurrence. Strategies to minimize pelvic recurrence including preoperative radiation and the principle of careful mesorectal excision to maximize the achievement of negative radial resection margins and negative residual disease are recommended. Also needed are standards for evaluating radial margins and lymph nodes to improved pathology reports.
Rectal cancer outcomes depend on the stage of the tumour, adequate surgical resection and appropriate use of adjuvant chemotherapy and radiotherapy. To compare our rectal cancer outcomes to those of other centres, we chose to review the charts of patients treated through the British Columbia Cancer Agency by combined chemotherapy and radiotherapy after curative surgical resection of adenocarcinoma of the rectum.
To do this we first determined the overall 5-year disease-specific survival. Second, we determined whether 5-year disease-specific survival and pelvic recurrence were influenced by tumour stage, nodal status, type of surgical procedure and the presence of residual disease; we also determined whether survival was affected by pelvic recurrence. Third, since adequate pathology reporting is necessary for accurate reporting of outcomes, we assessed the completeness of pathology reporting as contributing to understaging of residual disease.
Our hypothesis with respect to long-term outcome is that achievement of negative radial resection margins using the principles of mesorectal excision will result in decreased residual disease and improved survival. Our intention in this study is to review factors affecting survival and pelvic recurrence in order to design strategies for improved outcome. We chose the selected subset of patients since they are predicted to have significant mortality and pelvic recurrence and could, potentially, benefit from the results of this study.
Methods
We carried out a chart review of consecutive patients treated between Jan. 1, 1985, and Dec. 31, 1994, at the British Columbia Cancer Agency by postoperative chemoradiation after surgical resection for cure. We excluded patients who underwent palliative chemoradiation for known pelvic recurrence. Dukes’ stages A and D tumours were excluded because patients with Dukes’ A tumours were not given postoperative chemoradiation and those with Dukes’ D tumours were not treated for cure. In addition, we studied only patients whose histologic diagnosis was adenocarcinoma.
The chemoradiation protocol used was similar to that of other published studies.1,2 The primary tumour bed and the surrounding posterior pelvis were irradiated by megavoltage x-ray by a 3-field or 4-field technique. A boost field to the primary tumour bed was optional if small bowel could be excluded from the boost volume. A median isocentre dose of 45 Gy was given with a range of 41 to 60 Gy. Bolus 5-fluorouracil (5-FU)-based chemotherapy was given before, during and after radiotherapy.
Data collected
We recorded age, sex, 5-year disease-specific survival, 5-year pelvic recurrence, pathologic stage (Dukes, TNM), type of surgical procedure and presence of residual disease. Presence of residual disease was assessed from pathology and operative records. Pathology reports were reviewed for completeness with respect to assessment of distal and proximal resection margins, radial resection margin, and number of lymph nodes examined. Clinical status as of June 30, 1996, was determined from British Columbia Cancer Agency charts or by telephone contact with the family physician or the patient.
Analysis
Actuarial statistics on disease-specific survival and pelvic recurrence were calculated by the Kaplan–Meier method. Differences in the survival curves were examined using the logrank test. Cox’s proportional hazards regression model was used to examine the independence of the prognostic factors. All computations were done using SPSS for Windows, Release 7.5.1 (SPSS, Chicago, 1996).
Findings
During the study period, the charts of 191 patients were identified as satisfying the inclusion criteria for this study. There were 126 men (65%) and 65 women (35%). The median age was 63 (range from 19 to 83) years. The median follow-up was 39 (range from 3 to 132) months. The median potential follow-up — the date of the most recent follow-up minus the date of diagnosis — was 71 months.
The pathologic stage of tumours are given in Table 1: there were 64 Dukes’ B and 127 Dukes’ C tumours. A small number of T4 lesions were not associated with lymph-node metastases. Also, a small number of T1–2 tumours were associated with nodal metastases.
Pathologic Tumour Staging in 191 Patients Having Adenocarcinoma of the Rectum
Three types of surgical procedure were performed: anterior resection in 60 patients, abdominoperineal resection in 129 patients and Hartmann’s resection in 2 patients. The height of the tumour from the anal verge was not recorded as this information could not reliably be extracted from the records.
In 177 patients, there was no residual disease after surgery. However, 12 patients had microscopically positive margins and were assessed as having microscopic residual disease. In 2 patients, the macroscopic margin was positive and these 2 patients were assessed as having macroscopic residual disease.
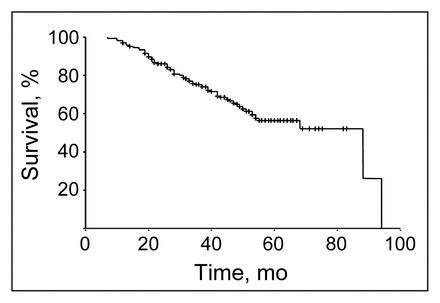
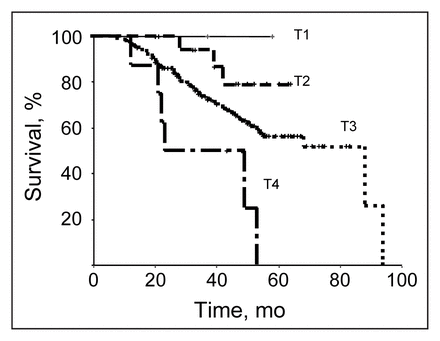
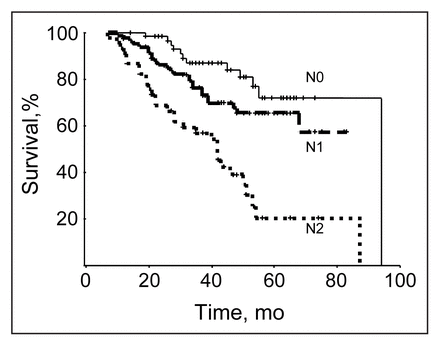
The overall 5-year disease-specific survival was 60% (Fig. 1). The effects of prognostic variables — tumour stage, nodal status, type of surgical procedure and presence of residual disease — are shown in Figs. 2 to 5. Typical relationships were observed, relating decreasing survival to increasing tumour stage and increasing nodal status (Figs. 2 and 3).
Disease-specific survival for all 191 patients with adenocarcinoma of the rectum who underwent resection followed by chemoradiotherapy for cure. At 5 years the rate was 60%.
Disease-specific survival in the 191 patients was significantly affected by tumour stage (p < 0.02). TNM classification.
Disease-specific survival for the 191 patients was significantly affected by nodal status (p < 0.001). TNM classification.
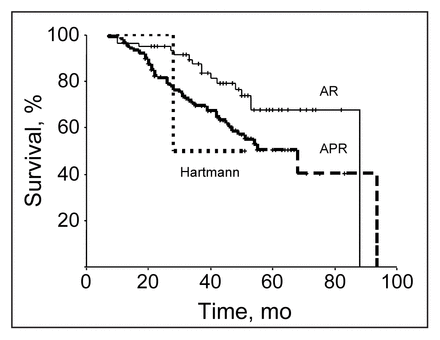
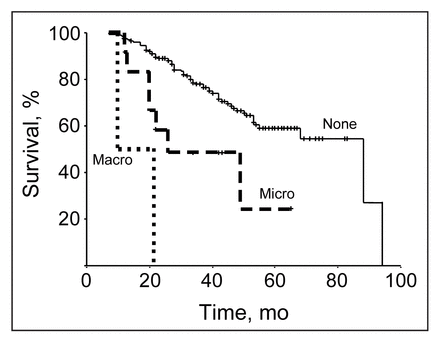
The type of surgical procedure affected outcome as indicated by a 70% 5-year survival for patients who had anterior resection compared with a 50% 5-year survival for those who had abdominoperineal or Hartmann’s resection (Fig. 4). The presence of residual disease also affected outcome. Neither of the patients who had macroscopic residual disease were 5-year survivors, 20% of those who had microscopic residual disease survived 5 years, and 60% of patients who did not have residual disease survived 5 years (Fig. 5).
Disease-specific survival in the 191 patients was significantly affected by type of surgical procedure (p < 0.04). AR = anterior resection. APR = abdominoperineal resection. Hartmann = Hartmann’s resection.
Disease-spectific survival is significantly affected by the presence of residual disease (p < 0.02). Macro = macroscopic residual disease, Micro = microscopic residual disease, None = no residual disease.
In a Cox’s proportional hazards regression multivariate analysis, all of the prognostic variables tested had a significant effect on 5-year survival: nodal status (p < 0.001), presence of residual disease (p < 0.017), type of surgical procedure (p < 0.036) and tumour stage (p < 0.038).
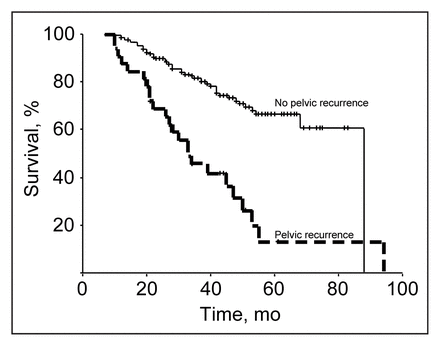
Five-year survival was significantly related to pelvic recurrence (p < 0.0001): 65% for patients who did not have pelvic recurrence versus 15% for patients who did (Fig. 6). The 5-year pelvic recurrence rate overall was 25%. Using a Cox’s proportional hazards regression multivariate analysis, we found that only residual disease was significantly related to pelvic recurrence (p < 0.001); tumour stage (p < 0.14), nodal status (p < 0.37) and type of surgical procedure (p < 0.20) did not affect pelvic recurrence.
Disease-specific survival in the 191 patients making up the study was significantly affected by pelvic recurrence (p < 0.001).
Of the 191 patients, pathology reports for 187 were reviewed. Proximal and distal resection margins were assessed in 180 (96%) of the 187 patients. However, only 83 (44%) of 187 reports contained an assessment of radial resection margins. The number of lymph nodes sampled ranged from 0 to 34 (median 6).
Discussion
The overall 5-year disease-specific survival rate was 60%. We found that survival was influenced by tumour stage, nodal status, type of surgical procedure and presence of residual disease after surgery. However, the strongest predictor of survival was pelvic recurrence. Overall this rate was 25%. Pelvic recurrence was affected by the presence of residual disease but not by tumour stage, nodal status or type of surgical procedure. Pathology reporting did not assess radial resection margin in the majority of cases and a median of 6 lymph nodes were sampled per case.
We compared the outcomes in our review with 2 well-known studies in which postoperative rectal cancer patients were treated with similar regimens of chemoradiation for cure.1,2 The Gastrointestinal Tumor Study Group performed a multiple-armed study of postoperative adjuvant chemotherapy or radiotherapy, or a combination of these.1 In the group of 46 patients who received postoperative chemoradiation, the 5-year survival was 70% and 5-year pelvic recurrence rate was 11%. The North Central Cancer Treatment Group reported a 5-year survival of 70% and 5-year pelvic recurrence of 14% in 104 patients treated with postoperative chemoradiation.2 Our review revealed a slightly poorer outcome than in these 2 studies.
Since the patient inclusion criteria and chemoradiation protocols for these studies were similar to ours, we suggest that possible factors influencing the difference in outcome are an inadequate surgical procedure and understaging of residual disease at the radial margins or lymph-node metastases resulting from inadequate pathology reporting.
We were unable to reliably assess the technical aspects of surgical resection from the operative records. Anterior resection was performed in about one-third of patients and abdominoperineal resection in two-thirds. It is likely that anterior resection was performed for tumours in the upper third of the rectum and abdominoperineal resection for tumours in the middle and lower thirds. Current surgical resection techniques recommend resection of the rectum with excision of perirectal lymphatics and lymph nodes encompassed by the mesorectal fascial envelope. Total mesorectal excision is indicated to resect all perirectal lymph nodes for middle and distal third rectal cancers, but partial mesorectal excision 5 cm distal to the cancer may be performed for upper third rectal cancers. We suggest that the principles of mesorectal excision were not practised at the time the surgical resections were undertaken in this study, since MacFarlane and Heald’s benchmark study on excellent outcomes from total mesorectal excision was not published until 1993.3
Pathology reporting was inadequate. We judged that the radial resection margin was adequately assessed by the pathologist if either there was a specific comment about it in the gross or microscopic description or there was a comment about painting the surface of the bowel with silver nitrate. Therefore, tumours that had not invaded through the muscularis propria (T1 and T2) were considered to have received adequate assessement of the radial resection margin. Even with these very liberal inclusion criteria, only 44% of reports included assessment of radial resection margins, whereas proximal and distal resection margins were routinely included in the pathology reports. Also, the median number of lymph nodes reported in our study was 6. In contrast the TMN Committee of the International Union Against Cancer and the American Joint Committee on Cancer recommend that a minimum of 12 lymph nodes be sampled in order to accurately assess lymphnode status.4–6 We suggest that our pathology reporting is inadequate based on underreporting of radial resection margins and the low number of lymph nodes examined. As such, perhaps our outcomes were poorer in part because of underestimation of residual disease and nodal status.
Survival was affected by tumour stage, nodal status, type of surgical procedure and presence of residual disease. Survival curves for tumour stage and nodal status show typical inverse relationships of increasing stage and nodal status to worsening survival. Although survival was better for anterior resection than abdominoperineal resection, tumour stage and nodal status for anterior resection and abdominoperineal resection did not differ. The ratio of Dukes’ B to Dukes’ C tumours was 1:2 for both anterior resection and abdominoperineal resection.
We propose 2 potential reasons why survival after anterior resection was better than after abdominoperineal resection. First, achievement of negative radial resection margins is technically more difficult for low tumours treated by abdominoperineal resection than for upper third tumours treated by anterior resection. During rectal cancer resection, the mesorectal fascial envelope encompassing the cancer and perirectal lymph nodes is easier to maintain intact above the peritoneal reflection than below because of adherence to adjacent anterior and lateral organs, nerves and blood vessels within the narrower distal pelvis. Evidence in support of increasing technical difficulty to achieve negative resection margins with more distal tumours was the presence of residual disease in 9% of abdominoperineal resections compared with 5% of anterior resections. Second, is the occurrence of residual disease in iliac lymph nodes. Metastasis to iliac lymph nodes is more frequent for lower third tumours that for upper third tumours of the rectum: 20% for low T3 versus 8% for upper T3 rectal cancers.7 Internal iliac lymphadenectomy was not performed in any of the resections in our study. As such, abdominoperineal resection as a surrogate for low height of the rectal cancer influences survival because of increased residual disease in iliac lymphatic vessels.
The presence of residual disease demonstrated a significant inverse relation to survival and to pelvic recurrence. This observation is similar to the observations from an 8-year series of patients having mesorectal excision for rectal cancer.8 Microscopic residual disease was successfully treated using postoperative chemoradiation in some of our patients, but macroscopic residual disease was not successfully treated in any case.
Pelvic recurrence was affected by residual disease but not by tumour stage, nodal status or type of surgical procedure. Our findings support the tenet that pelvic recurrence bears a direct relation to adequacy of surgical resection. Furthermore, pelvic recurrence affects survival more than any other predictive factor.
Our recurrence rate is higher than that reported in other studies. However, most other studies do not report on the presence of residual disease at the margins, which clearly affects outcome. Furthermore, most reports come from centres having technical expertise in rectal cancer surgery. In comparison, our outcomes involve rectal cancer surgery by surgeons whose expertise in surgery for rectal cancer varied widely. In all likelihood, our outcomes are comparable to those of other Canadian provinces whose surgeons have similar variation in expertise for rectal cancer surgery. Because our chemoradiation protocol is similar to that of published recommendations, we think it is less likely that the chemoradiation protocol is responsible for the increased recurrence rate in our rectal cancer patients.
Potential limitations of our study include a lack of statistical comparison of outcomes to other groups. The 191 patients reported in our study are consecutive patients referred to the British Columbia Cancer Agency for postoperative adjuvant chemoradiation for cure. Although there are approximately 600 new cases of rectal cancer in British Columbia each year, the referral rate to the British Columbia Cancer Agency for postoperative chemoradiation is just a fraction of the total number of new cases annually. In fact, of the 191 patients who received postoperative adjuvant chemoradiation, only 7 were referred in the period from 1985 to 1989. After the National Institutes of Health (NIH) consensus conference in 1990, the number of cases referred for adjuvant chemoradiation increased markedly. We did not exclude cases that met the inclusion criteria of postoperative adjuvant chemoradiation with curative intent. We did exclude cases of chemoradiation for palliation and cases in which postoperative radiotherapy was given without chemotherapy. The other important unknown is stage and outcome for nonreferred patients who had surgical management for rectal cancer without adjuvant therapy. Outcome data of nonreferred rectal cancer patients is not available in any database and is not reported in our study. Thus, the natural history of similarly staged patients in British Columbia is unknown, and outcomes from surgery alone are unknown. According to the British Columbia Cancer Registry, there is a slight gender preponderance for rectal cancer of 15.6 per 100 000 for men compared with 11.9 per 100 000 for women. We do not have an explanation for this. Possibly more men were referred for postoperative chemoradiation because of greater surgical difficulty in achieving clear margins in the more narrow male pelvis. Our referred patient cohort could be biased toward worse survival and greater pelvic recurrence. Alternatively, our patient cohort could have bias toward higher survival if similar staged patients were not referred for postoperative chemoradiation because of comorbid illness or inability to travel.
In summary, we recognize slightly worse outcomes for our patients relative to other centres. Therefore we need to recommend strategies to improve our outcomes. We recommend that we should strive to achieve negative radial resection margins in order to decrease pelvic recurrence and improve survival. Measures to aid achievement of negative radial resection margins include preoperative radiotherapy.9 Currently, we recommend a short course of 1 week of preoperative radiotherapy for mobile tumours of stages II and III (T3, N1–2),10 and full-course preoperative chemoradiation of 5 weeks for clinically fixed tumours.9 Our recommendations for increased use of preoperative neoadjuvant chemoradio therapy or radiotherapy are based on published studies and NIH guidelines. A definitive study comparing short-course preoperative radiotherapy to radical preoperative chemoradiotherapy is pending. Further, we fully support the principle of rectal cancer and nodal excision within an intact mesorectal fascial envelope.3 The recommendation that rectal cancer be excised with clear margins is supported by data from our study and from many other publications. Lastly, we need to improve pathology reporting to include assessment of the radial resection margins and examination of at least 12 lymph nodes.4–6 The surgeon and pathologist should base their assessment of the presence of residual disease on gross and microscopic examination of the resected specimen. Although improved pathology reporting will not improve outcome per se, greater attention to surgical margins will improve outcome. We suggest that accurate pathology reporting is required for correct interpretation of outcome and that accurate pathology reporting in publications of outcome should not be taken for granted.
Footnotes
Presented in part at the annual meeting of the Royal College of Physicians and Surgeons of Canada, Montreal, Que., Sept. 25, 1999.
- Accepted June 21, 2000.