Abstract
Background: Emerging data suggest asymptomatic gastrointestinal stromal tumours (GISTs) of the upper gastrointestinal (UGI) tract are not uncommon. We sought to determine their incidence in patients undergoing resection for UGI neoplasms and their impact on surgical and adjuvant treatment.
Methods: We accessed a database prospectively listing all patients undergoing resection of non-GIST neoplasms of the stomach and esophagus at a single university centre over a 4.5-year period and reviewed pathology reports for the presence of synchronous GISTs in the UGI tract. We compared patient demographic and tumour characteristics, operative procedures and postoperative outcomes.
Results: In all, 207 patients undergoing gastrectomy or esophagectomy for non-GIST neoplasms were included. We identified 15 synchronous GISTs in the UGI tract of 11 (5.3%) patients (1 preoperatively, 4 intraoperatively and 10 on final pathology), with an average age of 67 years. Most patients were men. Additional resections were required for GISTs identified pre- or intraoperatively. Final pathology revealed completely resected c-kit positive tumours of an average size of 0.5 (range 0.1–4.0) cm with low or very low risk of malignant potential. No patients received adjuvant therapy for the GISTs. After a median follow-up of 11 (range 2–36) months, 5 patients died from their primary cancer, 3 were alive with primary cancer recurrence, and 3 were alive without disease. No patients experienced GIST recurrence.
Conclusion: Incidentally finding a synchronous GIST during resection of UGI neoplasms is not uncommon; it may alter surgical treatment but is unlikely to impact long-term survival.
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract, accounting for 0.1%–3.0% of all gastrointestinal malignancies.1–3 These tumours are typically discovered in symptomatic patients (e.g., gastro-intestinal bleeding, abdominal pain) or incidentally in asymptomatic patients (e.g., diagnostic tests, during abdominal surgery, present in surgical specimens). The most common site of GISTs is the stomach, followed by the small intestine, colon, rectum and esophagus.3–5 Diagnosis of a GIST is confirmed immunohistochemically using anti-CD117 (c-kit) antibodies, since nearly all GISTs are c-kit positive.1,4 The malignant potential of a GIST is determined mainly by its size and mitotic count.1,6
Emerging data suggest that asymptomatic GISTs of the stomach are not rare. In a series of 100 consecutive total gastrectomies for gastric cancers, Kawanowa and colleagues7 reported 50 microscopic GISTs in 35% (35 of 100) of gastric specimens. These GISTs, however, were very small (greatest diameter 0.2–4.0 mm) with a very low risk of malignant potential (i.e., no mitosis), and they may represent a different clinical entity than gross GISTs. Abraham and colleagues8 reported a series of esophagectomies for esophageal cancers in which 18 incidental GISTs were found (greatest diameter 0.2–3.0 mm) in 10% (15 of 150) of esophagus specimens. Conversely, Liu and colleagues9 reported a large series of gastrointestinal epithelial malignant tumours in which less than 1% of surgical specimens contained synchronous GISTs. Thus, the incidence of synchronous GISTs is not yet clear. In addition, the influence of incidental synchronous GISTs on surgical and adjuvant therapy, to our knowledge, has not been studied. This is a potentially important issue, as the location of incidental GISTs can interfere with the ultimate choice of conduit to restore intestinal continuity after resection of the UGI neoplasms. Thus, we sought to determine the incidence of these tumours in patients undergoing resection for UGI neoplasms and their impact on surgical and adjuvant treatment in our institution.
Methods
After institutional review board approval, we accessed a database prospectively including all patients undergoing resection of non-GIST neoplasms of the stomach and esophagus at a single university centre between July 2005 and February 2010. Patients with previous esophageal or gastric resection, known synchronous tumour at another site and limited operative exposure to the abdomen were excluded. We reviewed operative and pathology reports for the presence of synchronous GISTs in the UGI tract (GIST group) among the included patients. Patient demographic characteristics, primary tumour locations, primary tumour characteristics, clinical staging and operative procedures were compared between the GIST group and patients without synchronous GISTs (non-GIST group). For the GIST group, time of discovery, GIST location, GIST characteristics, operative procedures and postoperative outcomes were reviewed.
Statistical analysis
We performed our statistical analyses using standard 2-tailed Student t tests and the χ2 test to compare means of the 2 groups and categorized data, respectively. We considered results to be significant at p < 0.05.
Results
From July 2005 to February 2010, 223 patients underwent esophageal or gastric resections at our institution. We excluded 16 of those patients (14 owing to previous gastric resections, 1 owing to synchronous colonic tumours and 1 owing to wedge resection of the esophagus only), leaving 207 patients for analysis. Most of the patients were men (73.4%), and the average age was 67 (range 22–86) years. Of these 207 patients, 83 had tumours located in the esophagus, 64 at the gastresophageal junction (GEJ) according to the Siewert classification and 60 in the stomach. Of the tumours, 161 were adenocarcinomas, 28 squamous cell carcinomas, 8 neuroendocrine carcinomas, 7 premalignant conditions, 1 adenosquamous carcinoma, 1 pulmonary inflammatory myofibroblastic tumour and 1 B cell lymphoma. The operative approach depended on the tumour location and patient performance status. Nevertheless, the abdomens of all patients in this study were exposed and examined intraoperatively (Table 1).
Patient demographic and clinical characteristics
Eleven patients (5.3%) were found to have a synchronous GIST in the UGI tract. There was no difference in age and sex between the GIST and non-GIST groups (Table 1). Owing to the large discrepancy in the number of patients in the GIST versus the non-GIST group, no statistical difference could be determined between the groups in terms of clinical status and tumour pathology.
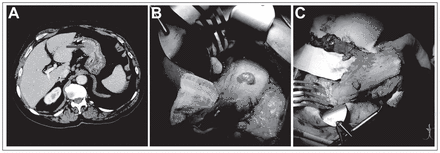
The 11 patients with synchronous GISTs had a combined total of 15 GISTs discovered: 1 preoperatively (Fig. 1A), 4 intraoperatively (Fig. 1B and 1C) and 10 on final pathology. Whereas 1 synchronous GIST was discovered preoperatively by gastroscopy and abdominal computed tomography (CT), the other 14 were undetectable preoperatively using endoscopy (0 of 14), CT (0 of 12), positron emission tomography (0 of 10) and/or diagnostic laparoscopy (0 of 2). Five (45%) patients with incidental GISTs were identified pre- or intraoperatively (Table 2); none had preoperative diagnostic laparoscopy. In these patients, the GISTs were located within the future gastric conduit. Consequently, additional resection was required to ensure complete excision of the incidental GIST. Additional wedge resections using gastrointestinal anastomosis (GIA) staplers were performed for patients 1, 4 and 9, in whom the synchronous GISTs were located in the body of the stomach. Patient 2 required only a simple excision using electrocautery since the GIST was very small and located on the serosal surface of the stomach. For patient 5 (Fig. 1A), the large submucosal GIST of the lesser curvature of stomach was exposed through a gastrostomy, and a wedge resection using GIA staplers was performed. Thus, the cases of 4 of these 5 patients (80%) substantially altered operative plans.
Incidental gastrointestinal stromal tumours in 2 patients undergoing open 3-hole esophagectomy. (A) Preoperative computed tomography with intravenous and oral contrast revealed a soft tissue mass within the lumen of the lesser curvature of the stomach (arrow) that required an additional excision intraoperatively (patient 5). (B) A large pedunculated exophytic mass on the anterior surface of the stomach (patient 1). (C) Presence of the large gastric mass shown in panel B on the gastric conduit, requiring a wedge resection. The calibre of the gastric conduit was subsequently reduced.
Demographic and clinical characteristics of patients in the GIST group
Of the 15 GISTs, 13 (87%) were found in the stomach and the other 2 (13%) in the esophagus. Most of them were small, with a median size of 0.5 (range 0.1–4.0) cm, and all of them had low mitotic counts (0–4 mitoses in 50 high-power fields [HPFs]), indicating a very low or low risk of malignant potential (Table 2). The clinically diagnosed GISTs were, however, significantly larger than those discovered solely on pathology (range 0.3–4.0 v. 0.1–0.6 cm, median 0.8 v. 0.3 cm, mean 1.4 v. 0.4 cm; all p < 0.05). The clinically diagnosed GISTs also had higher mitotic counts (range 2–4 v. 0–2 mitoses in 50 HPFs, median 2 v. 1 mitoses in 50 HPFs). None of these patients received additional adjuvant therapy for GISTs and none had GIST recurrence during the follow-up period (median 15 mo, range 2–36 mo). After the follow-up period, 5 patients had died from recurrence of their primary cancer, 3 were alive with recurrence of their primary cancer and 3 were alive without disease.
Discussion
We found 15 incidental GISTs in the UGI tract of 11 patients during esophagectomy or gastrectomy, leading to an incidence of 5.3% (11 of 207), which was much higher than previously reported by Liu and colleagues,9 who found an incidence of 0.74% (49 of 6585) among patients with UGI epithelial malignancies. Although the reason for this discrepancy is not known, differences in the patient demographic characteristics — a highly heterogeneous population in Montréal, Canada, versus a Chinese population in Sichuan Province, China — may have played a major role. We also found a significantly higher proportion of adenocarcinomas (78% [161 of 207] v. 64% [4203 of 6585], p < 0.001), especially in the esophagus (25% [51 of 207] v. 0.5% [35 of 6585], p < 0.001). Based on studies reported by Kawanowa and colleagues7 and Abraham and colleagues,8 incidental GISTs are preferentially located in the upper part of the stomach and GEJ of patients with UGI epithelial malignancies. Surgical specimens resected with distal gastrectomy for distal gastric adenocarcinomas or esophagectomy with minimal gastric resection for more proximal diseases may not include this entire UGI segment, hence decreasing the likelihood of identifying incidental GISTs. Likewise, surgical approach may influence the intraoperative detection of a synchronous GIST. Esophagectomy using a transthoracic approach without entering the abdomen may leave synchronous GISTs undetected intraoperatively at a more distal site. Since information regarding surgical procedures was missing in the report by Liu and colleagues,9 no comparison can be made between their results and ours. When extensive pathological examination with microscopic evaluation of the entire specimen was undertaken, Kawanowa and colleagues7 and Abraham and colleagues8 reported small GISTs in the surgical specimen in 35% and 10%, respectively, of patients undergoing resection of UGI epithelial cancer. Since most of the incidental GISTs were less than 1 cm in diameter, serial sections skipping a depth greater than 1 cm could leave these tumours undetected. Thus, the detection rate greatly depends on the number of histological sections per specimen examined. This may be another plausible contributing factor leading to the apparent difference. Based on our results and those of other published studies, incidental GISTs can be found frequently, and their prevalence is likely dependent on the detection methods and perhaps on the patient population.
Previous studies reported low rates of preoperative and intraoperative detection of incidental GISTs (0%–6%),7–9 whereas the intraoperative detection rate in the present study was 33% (5 of 15 incidental GISTs). The GIST size in the present series was not significantly larger than that reported by Liu and colleagues,9 but was much larger than those reported by Kawanowa and colleagues7 and Abraham and colleagues.8 Nevertheless, those identified pre- or intraoperatively in all these studies, including ours, were present either on the serosal or intraluminal surface, which could have been easily detected by preoperative diagnostic laparoscopy or gastroscopy, respectively. Thus, location rather than size of the incidental GISTs determined their intraoperative detection. The higher intraoperative detection rate in our study might only have been due to coincidence.
With respect to pre- or intraoperative identification of incidental GISTs in the context of a primary UGI epithelial malignancy, alteration of surgical therapy must be considered. If the decision is to remove the incidental GIST, surgical outcome may differ in 2 ways. First, obtaining a negative margin for the GIST can compromise the integrity of the gastric conduit depending on its size and location. Second, although the rate of staple line leak varies in the literature depending on the techniques and materials used, any additional staple line theoretically increases the chance of postoperative leak. By contrast, leaving an incidental GIST behind is associated with a risk of tumour progression, although these incidental GISTs all had a low risk of malignant potential in our study and in other published series.7–9 In addition, residual incidental GIST may be mistaken for recurrence or metastasis of the primary epithelial malignancy at follow-up. Consequently, patients may be subjected to unnecessary additional therapy when no recurrence truly exists. In light of these possibilities, it has been recommended that incidental GISTs be removed en bloc with other tumours when possible. Alternatively, local resection should be performed.10–12 Owing to the presence of incidental GIST, 5 (45%) patients with primary epithelial malignancies in our series required additional procedures intraoperatively. Simple excision or wedge resection was performed in all cases involving the eventual gastric conduit used to restore intestinal continuity. No cases of anastamotic leak were observed in this group of patients. However, because of the likelihood of much slower progression of incidental GIST relative to the primary epithelial malignancies in patients with gastroesophageal cancers in our study and in the literature, and given the poor prognosis associated with the primary epithelial malignancy in these patients (45% of patients died of recurrence of their primary cancer during the follow-up period of 6 to 36 mo), we suggest that excision of incidental GISTs be carried out only if this additional resection does not compromise the integrity of the gastric conduit and the intestinal continuity.
Surgical resection remains the treatment of choice for localized GISTs. Currently, no evidence exists supporting the use of adjuvant or neoadjuvant therapy in the treatment of non–high risk GISTs in which surgical resection is possible.13 All incidental GISTs found in our series and in the published reports7–9 were determined to have low or very low risk of malignant potential. None of the patients in our series or that by Liu and colleagues9 had GIST recurrence despite the lack of adjuvant therapy for GISTs. These patients typically died of a recurrence of their primary epithelial cancer because most of them had stage II to IV disease. Because prognosis is greatly dictated by the primary epithelial cancers rather than the GISTs, adjuvant therapy should be focused primarily on the more aggressive disease.
Conclusion
Finding a synchronous GIST in patients undergoing resection of UGI neoplasms is not uncommon, and its detection may depend on its anatomic location, surgical approach and pathological evaluation. The presence of these incidental GISTs can influence the surgical therapy and may impact the surgical outcome. However, given their low risk of malignant potential, the probability of GIST recurrence is far inferior to that of the primary cancer. Thus, one would have to be cautious not to overtreat these incidental GISTs and worsen the optimal surgical therapy and oncological outcome of the primary disease.
Footnotes
This manuscript was presented at the 51st Society for Surgery of the Alimentary Tract Annual Meeting at Digestive Disease Week, May 1–5, 2010, in New Orleans, Lo.
Competing interests: None declared.
Contributors: C.H.F. Chan and L. Ferri designed the study. C.H.F. Chan, J. Cools-Lartigue and V.A. Marcus acquired the data. C.H.F. Chan and J. Cools-Lartigue wrote the article, which V.A. Marcus, L.S. Feldman and L.E. Ferri reviewed. All authors analyzed the data and approved the article for publication.
- Accepted July 25, 2011.