Abstract
Background: Patients with extreme obesity are at high risk for adverse perioperative events, especially when opioid-centric analgesic protocols are used, and perioperative pain management interventions in bariatric surgery could improve safety, outcomes and satisfaction. We aimed to evaluate the impact of intraperitoneal local anesthesia (IPLA) on enhanced recovery after bariatric surgery (ERABS) outcomes.
Methods: We conducted a prospective double-blind randomized controlled pilot study in adherence to an a priori peer-reviewed protocol. Patients undergoing laparoscopic Roux-en-Y gastric bypass surgery (LRYGB) with an established ERABS protocol between July 2014 and February 2015 were randomly allocated to receive either IPLA with 0.2% ropivacaine (intervention group) or normal saline (control group). We measured pain scores, analgesic consumption and adverse effects. Functional prehabilitation outcomes, including peak expiratory flow (PEF) and the Six Minute Walk Test (6MWT) and Quality of Recovery Survey-40 (QoR-40) scores, were assessed before surgery, and 1 day and 7 days postoperatively.
Results: One hundred patients were randomly allocated to the study groups, of whom 92 completed the study, 46 in each group. There were no statistically significant differences between the 2 groups in baseline characteristics or any primary or secondary outcomes. Pain scores and analgesic consumption were low in both groups. There were no adverse events. Significant declines in PEF and 6MWT and QoR-40 scores were noted on postoperative day 1 in both groups; the values returned to baseline on postoperative day 7 in both groups.
Conclusion: Intraperitoneal local anesthesia with ropivacaine did not reduce postoperative pain or analgesic consumption when administered intraoperatively to patients undergoing LRYGB. Standardization of the ERABS protocol benefited patients, with functional prehabilitation outcomes returning to baseline postoperatively. Trial registration: ClinicalTrials.gov no. NCT 02154763
Implementation of Enhanced Recovery After Surgery (ERAS) in a wide variety of surgical models is well established and has shown the importance of multimodal strategies in improving perioperative patient safety and outcomes. 1 Patients undergoing elective weight loss (bariatric) surgery are at risk for increased complications owing to their obesity-related comorbidity. Enhanced recovery after bariatric surgery (ERABS) may be critical in this context for a variety of clinical reasons.2 For example, if ERABS interventions can improve the patient’s breathing and coughing ability, and ensure early oral intake or adequate ambulation, these outcomes could substantially reduce the risk of the major causes of morbidity and mortality related to bariatric surgery, including cardiorespiratory complications, deep vein thrombosis and pulmonary embolism.3
Pain management remains particularly challenging in patients with extreme obesity. When coexisting cardio-respiratory illnesses and the high prevalence of obstructive sleep apnea are combined with poorly or inappropriately managed pain, further perioperative complications can occur in this patient population.3 Traditional opioid-centric pain management protocols increase sedation, respiratory depression, nausea and vomiting, all of which can adversely affect recovery, early discharge and return to baseline function. 3 Effective perioperative pain management is one of the cornerstones of ERABS. Early recovery after bariatric surgery provides the framework to improve patient safety and outcomes with protocol standardization and multimodal interventions.2,4 Implementing multimodal nonopioid analgesia protocols and exploring regional anesthesia techniques are therefore important to achieving ERABS outcomes. These advances in perioperative care can potentially be applied to the wider population of patients with extreme obesity undergoing a variety of surgical procedures.4
Intraperitoneal local anesthesia (IPLA) has been studied extensively in nonbariatric general surgery and gynecology. 5,6 In bariatric surgery, IPLA with bupivacaine has been reported to reduce pain and analgesic consumption. 7,8 We conducted a prospective double-blind randomized controlled pilot study to evaluate the clinical efficacy of adding IPLA using ropivacaine to a standardized ERABS protocol9 to improve pain management and functional outcomes in patients undergoing elective bariatric surgery. We chose to study ropivacaine because it may be clinically more efficacious and have a better safety profile in locoregional anesthesia than bupivacaine.10,11
Methods
The research protocol was approved by the Ottawa Health Science Network Research Ethics Board (OHREB 20120743–01H) and registered with ClinicalTrials.gov (NCT 02154763) as a randomized double-blind placebo-controlled trial. The protocol for the trial was then peer reviewed and published a priori.9 Approval for the use of the study drug (ropivacaine) as an IPLA was deemed off-label. We obtained federal health care authority approval for the study (Health Canada no. 160184) before patient enrolment.
Participants
Patients undergoing laparoscopic Roux-en-Y gastric bypass for obesity at the regional Bariatric Centre of Excellence (The Ottawa Hospital) between July 2014 and February 2015 were approached by a participating surgeon or a nurse from The Ottawa Hospital Bariatric Surgery Clinic for participation. If the patient agreed to participate, consent was obtained by a member of the research team (A.J.) who was independent from the clinical care of patients.
We included all adults (age > 18 yr) who were able to tolerate general anesthesia and pneumoperitoneum, and to provide informed consent for the surgery. Patients with chronic pain requiring preoperative opioids were included. Exclusion criteria were planned sleeve gastrectomy (intraoperative conversion to sleeve gastrectomy after IPLA delivery was included and analyzed with the intent-to-treat approach); allergy to local anesthetics; severe underlying cardiovascular disease, including congestive heart failure, conduction abnormalities and ischemic heart disease; chronic renal disease stage 3 or higher (defined as creatinine clearance < 60 mL/h); hepatic dysfunction Child–Pugh class B or C; and previous foregut surgery, including esophageal, gastric, liver or pancreas resection. Inclusion and exclusion criteria are described in detail in the published protocol.9
The sample size for pilot trials is typically determined pragmatically. We aimed to randomly allocate 100 patients to the study groups.
Randomization
Patients were assigned in a 1:1 ratio to either the intervention (IPLA) group or the control group by means of a simple computer-generated in-hospital randomization platform. The unblinded data were kept securely by pharmacy and were generated only a day before the operating day for each patient. The study was adequately and appropriately blinded: patients and providers (surgeons, anesthesiologists, operating room staff, nurses on both preand postsurgery units) involved in data collection and analysis had no knowledge of the group to which the patient was allocated.
The pharmacy independently prepared the treatment solution (ropivacaine or normal saline) in a standardized 100 mL bag. The treatment solution was attached to the patient’s unique identifier and did not indicate to which study arm the patient was allocated. The intravenous bags were labelled according to Health Canada regulations and did not disclose the bag content. The treatment medication was delivered to the operating room on the day of surgery.
Intervention
All procedures were performed by 1 of 3 participating expert surgeons (J.M., J.-D.Y. or A.N.). All patients received acetaminophen (975 mg orally) and celecoxib (400 mg orally) 2 hours before surgery. With standard monitoring, anesthesia was induced with propofol and fentanyl, administered intravenously and a neuromuscular blocking agent was administered to facilitate orotracheal intubation and controlled ventilation. After induction of anesthesia, all patients received ketamine (20 mg) and dual antiemetic therapy (dexamethasone 8 mg, and ondansetron 8 mg) intravenously. Anesthesia was maintained with volatile agents via controlled ventilation using an air–oxygen mixture. A dexmedetomidine infusion (0.4–0.7 μg/kg/h) was continued throughout anesthesia, and additional boluses of fentanyl were administered as required. No other anesthetic or analgesic infusions were administered intraoperatively.
A standardized surgical technique was used for this study. After the pneumoperitoneum was created and all trocars were placed, the standard suction/irrigation device was used to instill 100 mL of 0.2% ropivacaine or normal saline into the peritoneal cavity at the start of the case and before surgical dissection. Under direct visualization, 50 mL of ropivacaine or normal saline was infused over the esophageal hiatus, and the remaining 50 mL was infused throughout the abdomen. The remainder of the surgery and anesthesia followed standardized procedures and protocols. For postoperative pain control, patients were offered, on demand, acetaminophen, followed by ketorolac, then tramadol, and finally hydromorphone.
Outcomes
Participants provided their baseline demographic information, existing comorbidities, past medical and surgical history, medications, allergies and social history. Baseline function and peak expiratory flow (PEF) were measured, and the Six Minute Walk Test (6MWT) and Quality of Recovery Survey-40 (QoR-40)12 were administered. The primary outcome was postoperative pain as measured on a numeric pain scale ranging from 0 (no pain at all) to 10 (worst pain imaginable). We considered a 30% reduction in pain in the intervention group compared to the control group a clinically significant improvement. The secondary outcomes included opioid analgesic use during the hospital stay, PEF, 6MWT result, impact on condition-specific quality of life (assessed with the QoR-40) and perioperative complications.
Statistical analysis
Data were collected as described in the study protocol9 and entered into a secure monitored database. We performed a multivariate analysis with the model adjusted for covariates such as age, gender, body mass index, pain syndromes and history of musculoskeletal diseases; we considered alcohol consumption, medication use and intraoperative concurrent procedures as time-fixed covariates. We described quantitative variables by mean and standard deviation (SD). Analgesic use was recorded in the postanesthesia care unit and at fixed time intervals until discharge. We analyzed repeated measures using a mixed modelling approach and performed an intention-to-treat analysis comparing the intervention and control groups for pain score, PEF, 6MWT score and QoR-40 score over time using SPSS version 18 for Windows (IBM Corp.).
Results
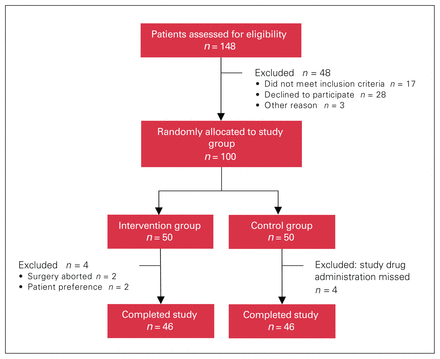
A total of 148 patients were approached, of whom 100 were randomly allocated to the study groups. Ninety-two patients completed the study, 46 in each group (Figure 1). There were 77 women (84%) and 15 men (16%), with a mean body mass index of 47.2 (SD 6.4) and a mean age of 44.8 (SD 9.3) years. The preoperative characteristics and intraoperative variables for the 2 groups are presented in Table 1. There was no statistically significant difference between the 2 groups except for mean body mass index and history of asthma, which were higher in the intervention group than in the control group. The prevalence of chronic pain was higher in the control group.
Flow diagram showing patient allocation.
Demographic characteristics, comorbidities, preoperative body mass index and intraoperative variables for patients undergoing bariatric surgery with or without intraperitoneal local anesthesia
There was no statistically significant difference between the intervention group and the control group in pain scores at any postoperative time point (Table 2). The average pain scores remained consistently low for the entire study period.
Mean postoperative pain scores for the 2 groups
Analgesic consumption was similar for the intervention and control groups (Table 3). There was wide variation in the mean dosages of the analgesics used, without any overall statistically significant or clinically relevant differences.
Mean analgesic use in the first 24 h postoperatively for the 2 groups
There were no differences at baseline or on the first postoperative day between the 2 groups in PEF, 6MWT score or QoR-40 score (Table 4). All patients, irrespective of their group allocation, showed a decrease in these outcomes in the first 24 hours postoperatively, and then 6MWT and QoR-40 scores had recovered by the seventh postoperative day. There were no differences between the groups, and all patients showed a return to their baseline functional status on the seventh postoperative day.
Mean secondary outcomes at baseline and on postoperative days 1 and 7 for the 2 groups
Discussion
In this pilot study evaluating the clinical efficacy of adding IPLA to a standardized ERABS protocol, we found no significant differences between the patients who received IPLA and those who did not in pain scores, analgesic consumption (even though there were more patients with a history of chronic pain in the control group than in the intervention group), PEF on postoperative day 1, and 6MWT and QoR-40 scores on postoperative day 7.
The negative results of this clinical trial must be interpreted in the context of low pain scores and analgesic consumption in both groups. All the study patients, irrespective of their group allocation, received a standardized opioid-sparing anesthetic and analgesic protocol. Multi-modal analgesia itself reduces pain scores, opioid analgesic consumption and adverse effects after bariatric surgery.13 In addition, our standardized ERABS protocol included both ketamine and dexmedetomidine, which are individually known to reduce pain and minimize opioid analgesic consumption without increased sedation or respiratory depression. These are highly desirable features in patients with extreme obesity and especially after bariatric surgery. 13,14 Beyond their excellent analgesic properties, these agents can contribute to ERABS outcomes through reduction in opioid-related adverse effects, including sedation, respiratory depression, nausea and vomiting.13–15 Perioperative care that includes ERABS protocols and implements opioid-sparing (or opioid-free) anesthetic strategies is a major evolution in bariatric anesthesia.16 In this paradigm shift toward minimizing opioid use after bariatric surgery, the role of regional anesthesia techniques continues to need evaluation. Our study contributes to this area of research by confirming lack of benefit of IPLA in improving outcomes. Nonetheless, our study protocol and methodology will be useful to guide further research in perioperative pain management techniques in ERABS.
The results of this study also need to be considered in the larger context of being conducted in a university level tertiary referral hospital that is a provincially designated Bariatric Centre of Excellence. Our well-established bariatric surgical program has provided elective bariatric surgery to more than 7000 patients since 2009. As observed elsewhere with well-established ERAS centres, beyond a certain point, further reductions in traditional measures (e.g., length of stay, complications and readmission rates) may not be possible.17 Nevertheless, over time and through improvements in the protocols, our research and that of other investigators18 draw attention to the role of functional prehabilitation by working toward improving patient education, engagement and empowerment.
Our ERABS patient education includes both hard-copy booklets and online resources that are combined with personalized teaching through in-person sessions. All these provide the patient with the information required to participate in an ERABS program. Furthermore, using tools such as the 6MWT and PEF further engages patients and allows them to participate actively in their own care. We chose these 2 measures as, in our opinion, they best reflect the ERABS outcomes we would like all our patients to achieve before and after bariatric surgery, including deep breathing and adequate ambulation. Since patients are not blinded to these measures of their level of activity before surgery, they are motivated to achieve them after surgery. Finally, it is our impression that these simple functional outcome measures empower patients to achieve the central goal of ERABS, which is a return to baseline.
Although further research and implementation are required to standardize these and other ERABS tools, this study is an important first step in that direction. Overall, ERABS should improve patient safety and outcomes after bariatric surgery and will benefit patients, providers and health care systems.
Limitations
The study was initially designed as a pilot study, which is a limitation. However, we were able to recruit the appropriate number of patients and executed the of methodology successfully, and the results are strongly suggestive of lack of benefit.
Conclusion
In the setting of a standardized ERABS protocol, this prospective double-blind randomized pilot trial showed no additional clinical benefit of IPLA in patients undergoing bariatric surgery. This shows the need for alternative methods for pain control after bariatric surgery.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. A. Jarrar, R. Wu, A. Neville, J.-D. Yelle and J. Mamazza acquired the data, which A. Jarrar, N. Eipe, R. Wu and J. Mamazza analyzed. A. Jarrar and N. Eipe wrote the manuscript, which all authors critically revised. All authors gave final approval of the article to be published.
Funding: This study was funded by the Department of Surgery, The Ottawa Hospital.
- Accepted November 30, 2020.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.