Abstract
Background: In controlled donation after circulatory determination of death (DCD), it is common to administer premortem heparin to potential donors. This practice remains controversial because there is limited evidence for it and there is the possibility of inducing hemorrhage. To our knowledge, no previous studies have assessed the effects of heparin timing and dose on graft function.
Methods: We performed a multicentre cohort study of consecutive DCD donors and the recipients of their organs. Anticoagulation administration was considered early if given near the time of withdrawal of life-sustaining measures and late if delayed until the onset of donor hypoxemia (oxygen saturation < 70%) or hypotension (systolic blood pressure < 60 mm Hg or mean blood pressure < 50 mm Hg). The anticoagulation dose was considered high if it was 300 units/kg or greater.
Results: Donor anticoagulation data were available for 301 kidney, 75 liver and 46 lung recipients. Heparin was administered in 92% of cases and was most commonly withheld in donors with cerebrovascular causes of death (p = 0.01). Administration was late in 59% and the dose was low in 27%. Among kidney recipients, there were no significant differences in need for dialysis, glomerular filtration rate over the first year after transplantation or graft survival on the basis of whether or not the donor received heparin, the timing of heparin administration or the dose of heparin. Among liver recipients, alkaline phosphatase concentrations over the first year were significantly higher among recipients who received organs from donors to whom lower doses of heparin had been administered.
Conclusion: Premortem heparin is widely used in DCD cases, but there is variability in timing and dose, which was not associated with kidney outcomes in this study. Donor anticoagulation may have a greater impact in preventing biliary complications following liver transplantation.
The opportunity for organ donation is an important aspect of end-of-life care for some critically ill patients and their families.1–3 Transplantation improves the survival of recipients, enhances their quality of life and is cost-effective, and it is the preferred treatment for many patients with end-stage organ failure.4–8 In Canada, there are more than 4000 patients on transplant wait lists.9
Controlled donation after circulatory determination of death (DCD) accounts for a growing proportion of the organs from deceased donors. For kidney transplantation, rates of delayed graft function are higher with DCD than with donation after neurologic determination of death (NDD), but longer-term graft survival appears to be similar.10,11 For liver transplantation, DCD is associated with a higher rate of biliary complications, but outcomes have still proven to be favourable when donors are carefully selected.12,13 For lung transplantation, outcomes using DCD grafts appear to be comparable to those using NDD grafts.14,15 There is limited experience with heart transplantation, but early results appear promising.16,17
At many centres around the world, potential DCD donors routinely receive a large dose of intravenous heparin near the time of withdrawal of life-sustaining measures (WLSM), with the aim of attenuating graft thrombosis during the subsequent period of warm ischemic time (WIT). This practice is usually justified using the principle of double effect, whereby potential harm from anticoagulation is deemed acceptable because of presumed benefit to organ recipients.16,17 International guidelines vary; some recommend anticoagulation as standard care, while others are more neutral or consider it to be ethically problematic because of potential harm to the donor.18–25 The latter concern is heightened when intracerebral hemorrhage, subarachnoid hemorrhage or traumatic brain injury is the cause of death, since these are conditions where therapeutic anticoagulation is generally contraindicated. Furthermore, evidence for the efficacy of premortem donor anticoagulation at improving recipient graft function is limited, with favourable outcomes reported in some regions (e.g., United Kingdom) where it is rarely used.10,26–29 Administration of heparin before death remains a source of discomfort for some critical care providers.30,31
Canadian guidelines acknowledge that high-quality evidence for premortem anticoagulation is lacking and that there are scenarios where clinicians may consider it to be inappropriate.21,22 Nevertheless, observational studies demonstrate that more than 90 % of potential DCD donors in Canada receive intravenous heparin before death.32,33 There is, however, no consensus regarding the optimal timing of anticoagulation relative to WLSM and the most appropriate dose. Earlier administration and a higher dose could theoretically have a greater protective effect on graft function but also result in greater risk of iatrogenic hemorrhage. We performed a multicentre cohort study to assess the relationship between premortem anticoagulation practices and transplantation outcomes.
Methods
This was a multicentre retrospective cohort study assessing all consecutive DCD donors and the recipients of their organs in western Canada from 2008 to 2017. The study was performed in collaboration with BC Transplant, the Human Organ Procurement and Exchange (HOPE) Program (Northern Alberta), the Southern Alberta Organ and Tissue Donation Program, the Southern Alberta Transplant Program, the Saskatchewan Transplant Program and Transplant Manitoba. Research ethics boards individually approved the study in each of the provinces (REB 17-1095 at University of Calgary).
Consecutive DCD organ donors and transplant recipients were identified from the databases of each organ donation agency and transplant program, respectively. Potential donors were included if they underwent WLSM with the possibility of organ donation following death. From each donor record, we determined whether or not heparin was administered, as well as the dose and timing of administration in relation to WLSM, the onset of physiologic instability following WLSM (hypoxemia defined as sustained arterial oxygen saturation < 70 % and hypotension defined as sustained systolic blood pressure < 60 mm Hg or mean arterial pressure < 50 mm Hg) and death. We excluded donors for whom no information about heparin use was available.
Heparin administration was categorized as early if it occurred near the time of WLSM (before, concomitant with or shortly after WLSM; but before onset of hypoxemia or hypotension) and late if it occurred after the onset of significant hypoxemia or hypotension. Heparin dose was considered high if the donor received 300 units/kg or more, and it was considered low if the donor received less than 300 units/kg. This threshold was chosen because it is a standard dose used during cardiopulmonary bypass, which is sometimes cited as the rationale for similar dosing in DCD, even though it greatly exceeds the usual dose for therapeutic anticoagulation in other circumstances.34 We also assessed heparin timing and dose as continuous variables. Additional donor data included variables required to calculate the kidney and liver donor risk indices (KDRI and LDRI, respectively), both of which are measures of graft quality.35,36
Recipient data were extracted from electronic and paper-based medical records. Our primary analysis focused on kidney transplantation: outcomes of interest included recipient dialysis within the first week; estimated glomerular filtration rate (eGFR) at 1, 3, 6 and 12 months; and graft survival over time.37 In a secondary exploratory analysis, recognizing that the sample size would be smaller, we also assessed graft function in DCD liver and lung recipients. Outcomes in liver recipients included alkaline phosphatase and bilirubin concentrations (as surrogate measures for cholangiopathy) at 1 week and 3, 6 and 12 months; radiographic or biopsy evidence of cholangiopathy; and graft survival over time. Available outcomes in lung recipients included graft survival and intensive care unit (ICU) length of stay.
Statistical analysis
Categorical variables were quantified as proportions, and comparisons were performed using χ2 analysis or the Fisher exact test, as appropriate. Continuous variables were expressed as medians with interquartile ranges (IQRs) and compared using the Kruskall–Wallis test. Associations with quantitative variables (eGFR and liver chemistry) over the initial year following transplantation were assessed using mixed-effects models. Because eGFR, alkaline phosphatase concentrations and bilirubin concentrations are not necessarily linear over the weeks to months following transplantation, a quadratic term was added to models.
Multivariate analysis assessing associations between donor heparin timing or dose and recipient eGFR adjusted for KDRI, time from donor cardiac arrest to organ perfusion or functional warm ischemic time (time from onset of donor hypoxemia [arterial oxygen saturation < 70%] or hypotension [systolic pressure < 60 mm Hg or mean arterial pressure < 50 mm Hg] to organ perfusion) (separate models were created for each), cold ischemic time, use of ex vivo perfusion, and recipient age and sex. These variables were chosen a priori on the basis of known associations with graft function.35,36,38–41 Because of smaller sample size, multivariate models assessing recipient alkaline phosphatase and bilirubin concentrations adjusted only for LDRI and time from donor cardiac arrest to organ perfusion. Kaplan–Meier curves were used to assess graft survival stratified by heparin usage patterns and were compared using log rank tests. Analyses were performed with SAS version 9.4 (SAS Institute) and graphs were created using GraphPad Prism version 8. We considered p values less than 0.05 to be statistically significant.
Results
Anticoagulation practices in potential kidney donors
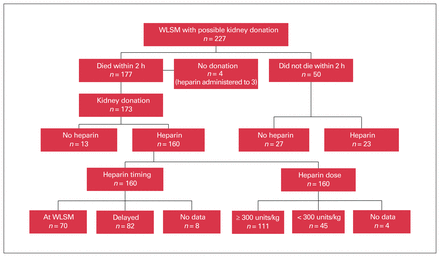
There were 227 patients who underwent WLSM with the possibility of subsequent DCD kidney donation, of whom 177 (78%) died within 2 hours and 173 donated at least 1 kidney (Figure 1). Heparin was administered in 160 (92%) cases. It was more likely to be withheld from potential donors with a cerebrovascular cause of death, including 29% of those with subarachnoid hemorrhage and 21% with intracerebral hemorrhage, compared with only 6% of those with hypoxic–ischemic brain injury and none with traumatic brain injury (p = 0.01) (Table 1).
Anticoagulation practices in potential kidney donors with circulatory determination of death. WLSM = withdrawal of life-sustaining measures.
Comparisons of kidney donor characteristics based on premortem anticoagulation practices
Data on timing of heparin administration were available for 152 of the 160 (95%) kidney donors in whom it was used. Heparin was given before, or at the same time as, WLSM in 70 of these 152 donors (46%), but it was delayed until the onset of either sustained hypoxemia or hypotension in 82 (54%) (Figure 1). In the former group, heparin was administered at a median of 0 (IQR 0–1) minutes after WLSM. In the latter patients, heparin was administered at a median of 9 (IQR 5–16) minutes after WLSM, but it was sometimes delayed by more than 60 minutes, if this was how long it took for physiologic instability to develop. Information about dosing was available for 156 of 160 (98%) patients; among these 156 patients, it was 300 units/kg or greater in 111 (71%) and less than 300 units/kg in 45 (29%) (Figure 1).
Kidney graft function in recipients
Data were available for 301 DCD kidney transplant recipients. There was no significant differences in the proportion of recipients who received dialysis during the first week following transplantation based on heparin timing (59% with early v. 56% with late, p = 0.67) or dose (54% with higher v. 65 % with lower dose, p = 0.10) or whether the donor had received any heparin (56% with heparin v. 45% without, p = 0.34). Similarly, when heparin timing and dose were assessed as continuous variables, they were not predictive of the need for early dialysis.
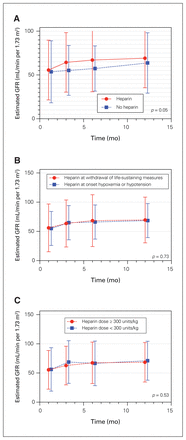
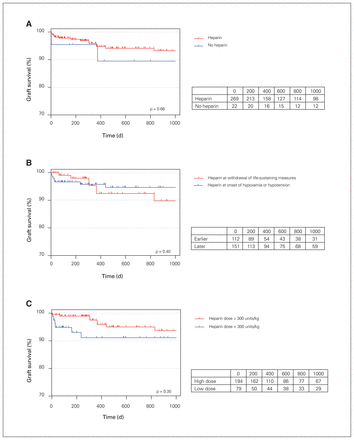
Figure 2 shows mean recipient eGFR over the first year after transplantation stratified by anticoagulation practices. There were no differences in eGFR based on heparin timing or dose. When heparin timing and dose were assessed as continuous variables, they were also not predictive of recipient eGFR (p = 0.59 and p = 0.16, respectively). Estimated GFR improved more rapidly over time following transplantation when heparin was given (p = 0.05), but there was no statistically significant difference in multivariable modelling (p = 0.83) or at any individual time point after transplantation. There was also no stastically significant difference in multivariate modelling for heparin timing (p = 0.85) or for heparin dose (p = 0.74). Figure 3 shows death-censored kidney graft survival stratified by heparin administration practices, again with no clear differences between groups. No patients were lost to follow-up within the first year.
Mean estimated GFR (with standard deviations) over the first year following kidney transplantation after circulatory determination of death, stratified according to (A) whether or not heparin was administered in the organ donor, (B) the timing of heparin administration, and (C) the heparin dose. GFR data were available for 292, 285, 277 and 274 patients at 1, 3, 6 and 12 months, respectively. GFR = glomerular filtration rate.
Death-censored kidney graft survival curves stratified according to (A) whether or not heparin was administered in the organ donor, (B) the timing of heparin administration, and (C) the heparin dose. Corresponding tables show the number of patients at risk based on the number of days post-transplant.
Liver and lung transplant outcomes
Donor heparin was administered to 66 of the 75 (88%) liver recipients. Of the 9 recipients who received an organ for which donor heparin had been withheld, none experienced graft loss during the first 3 years after transplantation and 1 was diagnosed with ischemic cholangiopathy. Alkaline phosphatase and bilirubin concentrations over the first year after transplantation (measured at 1 and 4 wk and 3, 6 and 12 mo) did not differ significantly at any time point among patients who received organs for which donor heparin had been given versus those who received organs for which it had been withheld.
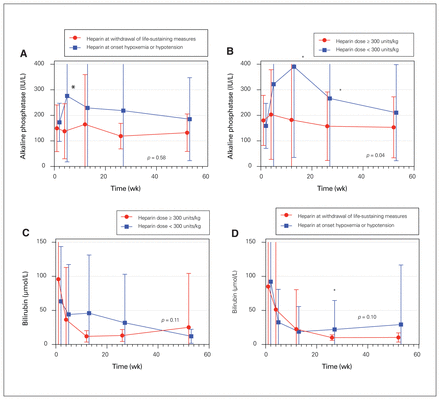
When heparin was administered in a delayed fashion, liver recipients had significantly higher mean alkaline phosphatase concentrations at 1 month (p = 0.004) and higher bilirubin concentrations at 6 months (Figure 4). Mixed effects models comparing earlier versus later heparin administration did not, however, show any statistically significant difference. Similarly, when time from WLSM to administration of heparin was modelled as a continuous variable, it was not predictive of recipient liver enzyme or bilirubin concentrations over the ensuing year.
Mean alkaline phosphatase (with standard deviations) over the first year following liver transplantation after DCD stratified according to (A) the timing of administration and (B) dose of heparin, and mean bilirubin concentrations (with standard deviations) over the first year following liver transplantation after DCD stratified according to (C) the timing of administration and (D) dose of heparin. Data were available for 71, 69, 67, 65 and 60 patients at 1 week, 1 month, 3 months, 6 months and 12 months, respectively. *p < 0.05 for comparison at that particular time point. DCD = circulatory determination of death.
Compared with higher dose heparin (≥ 300 units/kg), lower dose heparin was associated with higher alkaline phosphatase concentration at 3 months (p = 0.02) and 6 months (p = 0.04). This analysis was confounded by the observation that donors receiving lower dose heparin had longer time intervals from cardiac arrest to organ perfusion. Nevertheless, in both univariate (p = 0.04) and multivariate (p < 0.001) models, alkaline phosphatase concentrations were significantly higher over the first year after transplantation when lower dose heparin was used (Figure 4). This relationship persisted when heparin dose was considered as a continuous rather than dichotomous variable (p < 0.001 in multivariate analysis). When donor heparin dose was considered in tertiles, patients in the lowest dose group (heparin dose < 355 units/kg) had significantly higher alkaline phosphatase concentrations (p = 0.02), but there was no significant difference between the highest tertile (> 405 units/kg) and intermediate tertile (355–405 units/kg) groups (p = 0.43).
Radiographic or biopsy evidence of ischemic cholangiopathy was documented in 12 liver recipients (16%). This did not differ on the basis of the use of heparin (11% with heparin v. 15% without, p = 0.75), timing of administration (5% with early v. 17% with late, p = 0.19), or dose (14% with high dose v. 15% with low dose, p = 0.88). Similarly, the rate of cholangiopathy was not significantly influenced by heparin timing or dose when analyzed as continuous variables (median time from WLSM to heparin administration 5 [IQR 3–8] min with cholangiopathy v. 5 [IQR 0–10] min without, p = 0.91; median dose 364 [IQR 322–400] units/kg with cholangiopathy v. 378 (IQR 321–429) units/kg without, p = 0.44).
Only 3 of 46 patients received lung transplants from donors in whom heparin had been withheld; none died or experienced graft loss. Recipient ICU length of stay was similar regardless of the timing (5 [IQR 3–11] d with early v. 5.5 [IQR 2.5–10.5] d with late administration, p = 0.50) or dose (4 [IQR 3–8] d with high v. 7.5 [3.5–11] d with low dose, p = 0.44) of heparin administration.
Given the moderate sample size and small number of events, survival analysis for liver and lung transplantation is not presented in the main text, but it is available in Appendix 1 and Appendix 2 (available at www.canjsurg.ca/lookup/doi/10.1503/cjs.023120/tab-related-content). There were no statistically significant differences on the basis of anticoagulation practices.
Discussion
We observed significant variability in the use of premortem anticoagulation among potential DCD donors in western Canada. Heparin was given before death in more than 90% of potential donors. Although outcomes were generally favourable in recipients where donor heparin had been withheld, our analysis did not focus primarily on comparisons of graft outcome with or without donor anticoagulation. Rather, there was significant variability in timing and dose of heparin administration, the impact of which has not previously been reported, to our knowledge.
Complete avoidance of anticoagulation was more likely among potential donors with intracerebral hemorrhage or subarachnoid hemorrhage, and it was uncommon with other causes of death. This demonstrates that critical care physicians have concerns about anticoagulation causing harm in some potential DCD donors. Iatrogenic hemorrhage has not been reported in the literature, and it was not systematically sought in our database. However, if expansion or recurrence of intracranial hemorrhage were to theoretically occur in a potential donor, it is unlikely that it would be detected because computed tomographic scans are never repeated under these circumstances.
When heparin was given, it was commonly infused at some point after WLSM but before circulatory arrest. The rationale for this approach is that delaying anticoagulation until the potential donor is close to death minimizes the chance that it will induce or perpetuate substantial hemorrhage, while theoretically still allowing the drug to circulate to organs that will be recovered and transplanted. If potential donors do not develop physiologic instability within 1–2 hours following WLSM, anticoagulation (and any resultant potential for iatrogenic hemorrhage) can often be avoided altogether. We did not observe any significant differences in kidney transplant outcomes on the basis of the timing of donor heparin administration. Thus, delaying administration may be a helpful compromise in situations where transplant clinicians prefer that donors receive premortem anticoagulation, but critical care practitioners have reservations about doing so.
There is no consensus about the optimal dose of heparin in DCD.16–23 A typical loading dose when heparin is used for the treatment of thrombosis is 80 units/kg or less. A dose of approximately 300 units/kg is routinely used for cardiac surgery34 and is also the most common dose to be used for DCD, although some centres report using as much as 1000 units/kg.42 The theoretical rationale for such a high dose of heparin is to ensure that there is sufficient anticoagulation in graft microvasculature. In our cohort, the dose of heparin ranged from as low as 74 units/kg to as high as 667 units/kg. We did not detect any statistically significant differences in kidney transplant outcomes on the basis of the dose of heparin.
There are 2 previous human studies in the literature assessing the impact of premortem heparin administration on kidney transplant outcomes. Kamal and colleagues reported a single-centre comparison of 23 kidney transplants performed with heparin and 29 performed without and did not find any significant differences in delayed graft function, graft survival and creatinine clearance.43 Narvaez and colleagues assessed the Scientific Registry of Transplant Recipients (SRTR) and found that fewer than 5% of kidney transplants in the United States are currently performed in the absence of heparin, a figure that has declined markedly over the past 15–20 years from as high as 47% in 2003. There was, again, no significant difference in any relevant outcome on the basis of whether or not heparin was used.44 This lack of benefit from premortem anticoagulation in kidney transplantation is consistent with the observation of favourable outcomes reported in regions where anticoagulation is uncommon.10
Biliary complications from ischemic cholangiopathy are a particularly important source of morbidity in some recipients of DCD livers, such that donor anticoagulation may have a stronger rationale in liver transplantation.39,40,45 Our assessment of liver transplant outcomes was limited by the relatively small sample size. Nevertheless, our multivariate analysis demonstrated significantly higher concentrations of alkaline phosphatase when the dose of heparin was less than 300 unit/kg. This observation may suggest that inadequate donor anticoagulation predisposes liver recipients to ischemic cholangiopathy. This conclusion should be viewed as preliminary, because there was no clear detrimental effect in the 9 recipients who received transplants from donors from whom heparin was withheld altogether. Furthermore, although we did adjust for it in the multivariable analysis, donors treated with lower dose heparin tended to have longer time intervals from cardiac arrest to organ perfusion. The probable reason for this difference is that centres that used lower dose heparin also required electrical asystole (rather than only 5 min of absent pulse pressure) before the declaration of death. Although our data suggest a possible benefit from using at least 300 units/kg of heparin, we did not find any trend favouring even higher doses.
Narvaez and colleagues used the SRTR to assess DCD liver transplantation outcomes with or without heparin. Liver donors who did not receive heparin were, on average, older and (as in our study) more likely to have died of a cerebrovascular accident. In multivariate models, liver transplantation performed in the absence of donor heparin was associated with a higher rate of primary graft nonfunction and worse graft survival.46 However, withholding of heparin was far more common in an earlier era (2003–2007), during which graft survival rates were substantially worse, such that the apparent advantage of giving heparin may have been in large part due to increasing experience with DCD transplantation over time. Accordingly, 1-year liver graft survival when transplantation was performed in the absence of heparin was only 73%, which is markedly lower than contemporary results from the United Kingdom, where heparin is rarely used and the 1-year liver graft survival rate between 2001 and 2015 was 87%.47 Still, our findings support the contention that donor heparin may have a greater benefit in liver transplantation compared with kidney transplantation, particularly in relation to biliary complications. However, confirmatory studies are needed before definitive conclusions can be reached.
To our knowledge, no previous cohort studies have explicitly evaluated the use of donor heparin in DCD lung transplantation. However, a large international registry reported that donor heparin was used in only 53% of cases.15 As in the 3 recipients in our cohort whose donors did not receive any heparin, outcomes were largely favourable. In our study, we also did not observe any significant differences with early versus late heparin administration or with high versus low dose.
Numerous animal studies have assessed the importance of anticoagulation in DCD. In a porcine model of DCD liver transplantation using normothermic regional perfusion, Hessheimer and colleagues found that fibrin deposition in liver grafts was rare, regardless of whether or not heparin had been administered before cardiac arrest.48 However, heparin appeared to have other cyto-protective and anti-inflammatory effects that were associated with better graft function. In a porcine model of DCD lung transplantation, Sanchez and colleagues found that static lung compliance, pulmonary vascular resistance and gas exchange were better in lungs recovered from animals that had received 300 units/kg of heparin before cardiac arrest.49 These findings were not confirmed in a similar study by Liersch-Nordqvist and colleagues.50 In addition, Keshava and colleagues compared lungs when heparin was administered before versus after cardiac arrest and found no difference in occurrence of thrombosis.51
The main strength of our study is that it is population based, involving a consecutive series of all DCD donors and recipients in a defined geographic region, thereby avoiding selection bias. We were able to obtain very granular data regarding timing and dose of heparin.
Limitations
Important limitations of our study include its retrospective design, the relatively small number of liver and lung transplants and the fact that few donors did not receive any heparin. However, the numbers of patients who received earlier versus later heparin, or higher versus lower dose heparin, were more balanced. Future studies could assess whether anticoagulation has a greater impact in more “marginal” donors. The age and KDRI of our donors were comparable to those in other cohort studies.10,35 It is also currently unclear how potential innovations in DCD, such as ex vivo support or normothermic regional perfusion, might affect the need for anticoagulation.
Conclusion
Donor anticoagulation is widely used in DCD despite limited data to support its efficacy. We found no evidence that earlier heparin administration (near the time of WLSM) improves kidney graft outcomes compared with a strategy of delaying administration until it is clear that the patient will actually die within the requisite time frame for DCD to be possible. Similarly, we found no evidence that higher dose heparin is superior to more conventional anticoagulant doses in kidney donation and transplantation. In the context of liver transplantation, we observed somewhat lower alkaline phosphatase concentrations with administration of higher doses of heparin, suggesting a potential effect in ameliorating ischemic cholangiopathy. Overall, our findings suggest that there should be equipoise to study different approaches of administering premortem anticoagulation in future prospective clinical trials.
Footnotes
Competing interests: None declared.
Contributors: A. Kramer, P. Nickerson and L. Tibbles designed the study. K. Holliday, D. Kutsogiannis, P. Kim and A. Robertson acquired the data, which S. Keenan, G. Isac, D. Kutsogiannis and N. Kneteman, analyzed. A. Kramer and D. Kutsogiannis wrote the article, which K. Holliday, S. Keanan, G. Isac, D. Kutsogiannis, N. Kneteman, P. Kim, A. Robertson, P. Nickerson and L. Tibbles critically revised for important intellectual content. All authors gave final approval of the article to be published.
Funding: This work was funded by a grant from the Alberta Organ and Tissue Donation Agency, Alberta Health.
- Accepted August 10, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/