Abstract
Background: Open surgical and percutaneous endovascular procedures aimed at arresting traumatic life-threatening hemorrhage are usually performed in rapid serial fashion by surgeons and interventional radiologists; truly simultaneous procedures require modifications in technique, workflow and team collaboration. The primary objective of this study was to prospectively audit outcomes in patients with ongoing hemorrhage who underwent truly simultaneous open and percutaneous procedures.
Methods: We prospectively evaluated the cases of all severely injured patients who required an open and percutaneous procedure within the hybrid RAPTOR (resuscitation with angiography, percutaneous techniques and operative repair) suite at the Foothills Medical Centre, Calgary, Alberta, Canada, between Apr. 4, 2013, and Dec. 5, 2019. We compared outcomes between the truly simultaneous and rapid serial cases.
Results: Thirty-five patients (31 [89%] male, median age 46 yr, median Injury Severity Score 30, blunt mechanism in 26 cases [74%]) underwent a hybrid intervention in the RAPTOR suite to stop ongoing hemorrhage during the study period. Twenty-three patients (66%) had a rapid serial procedure, and 12 (34%) had a truly simultaneous procedure. Demographic characteristics were similar between the 2 groups. Compared to the rapid serial group, a higher proportion of patients in the truly simultaneous group were hemodynamically unstable (11 [92%] v. 13 [56%], p = 0.03) and required damage-control procedures (10 [83%] v. 12 [52%], p = 0.03). The time from hospital arrival to procedure initiation was shorter for the truly simultaneous group (mean 31 min v. 59 min, p = 0.02), and a lower proportion had initial radiologic studies (3 [25%] v. 16 [70%], p = 0.01). The median hospital length of stay, intensive care unit stay and mortality rate were similar between the 2 groups.
Conclusion: Truly simultaneous open and percutaneous procedures to stop ongoing hemorrhage were unique in both patient and procedural details. For the most severely injured patients, the provision of truly simultaneous modalities is necessary to achieve clinical outcomes equivalent to those of less ill patients.
Ongoing hemorrhage remains the dominant cause of death after severe injury.1–8 There is an increasing variety of instruments aimed at technical hemorrhage control and concurrent resuscitation.6,9 One of these paradigm-shifting concepts is the hybrid RAPTOR (resuscitation with angiography, percutaneous techniques and operative repair) operating environment.7,9–12 This tool allows a transition in care from a location-based approach to a truly disease- and urgency-based algorithm.11 Previous publications have shown that a subset of critically injured patients clearly benefit from access to a hybrid RAPTOR trauma suite, with reduced morbidity and mortality.7,11 Despite the substantial financial costs associated with RAPTOR technology,7,9–12 patients who require nearly concurrent emergent percutaneous and open procedures to arrest ongoing hemorrhage may be saved by this resource.7,11 Improving efficiencies for patients with continuous bleeding who would have traditionally required transportation between venues (angiography suite and operating room)7,9–12 has been transformational.
The terminology and technical details that surround truly hybrid operating environments remain heterogeneous and unclear. In numerous publications, the term “simultaneous” is used to describe the ability of clinicians to arrest ongoing hemorrhage and resuscitate patients in a single interventional location (i.e., RAPTOR suite).12 Like our oncologic colleagues,13 other authors have also used the terms “synchronous” or “concurrent.”7,11 The reality is that very few of these potentially life-saving trauma procedures are truly simultaneous. They are better described as “rapid serial” interventions (open surgical procedures followed immediately by percutaneous endovascular techniques, or vice versa) that are performed in the same location and during the same visit. These “combined” interventions do not occur at precisely the same time within the context of the overall procedure.
The primary goal of this study was to prospectively audit outcomes in patients with ongoing hemorrhage who underwent truly simultaneous/concurrent/synchronous open and percutaneous procedures. Our secondary objective was to describe the optimal procedural set-up and human flow factors for truly simultaneous RAPTOR interventions.
Methods
All adult (age ≥ 16 yr) patients who were severely injured (Injury Severity Scale [ISS] score ≥ 12) and required a hybrid intervention (open operative and percutaneous procedures) in the RAPTOR suite between Apr. 4, 2013, and Dec. 5, 2019, were evaluated prospectively. The Foothills Medical Centre, Calgary, Alberta, Canada, is a high-volume (> 1400 admissions per year) regional tertiary care, level I trauma referral centre with a catchment of about 3 million citizens from southern Alberta, southwestern Saskatchewan and southeastern British Columbia. All trauma team leaders are fellowship-trained trauma surgeons with extensive clinical experience. The percutaneous team usually consists of interventional radiologists who are also fellowship trained (vascular surgeons sometimes perform the aortic stent grafting component). All trauma procedures are performed in the RAPTOR suite, which is dedicated to trauma cases almost 24 hours a day, 7 days a week, with rare exceptions (including redundant hybrid capability in the adjacent cardiac hybrid suite whenever the trauma-dedicated RAPTOR suite is being serviced or is in use).
We classified patients into those who had truly simultaneous open and percutaneous interventions (i.e., 2 teams working in unison) and those who had rapid serial open and percutaneous procedures (i.e., 1 team followed by a second team) within the same setting. We noted patient and injury demographic characteristics, flow of care, specific interventions and patient outcomes.
As in our previous analyses,7,11 a panel of trauma surgeons, interventional radiologists and trauma clinicians was created to review each hybrid RAPTOR case. This panel used the definitions of “clear” and “potential” benefit. Use of the RAPTOR suite was deemed to show a clear benefit in cases in which the patient required a hybrid procedure and would have been extremely likely to have died if a truly simultaneous procedure had not been performed. We defined use of the RAPTOR suite as showing a potential benefit in scenarios in which an emergent hybrid procedure was required, but the absence of a truly simultaneous approach (i.e., rapid serial used) did not directly result in additional morbidity or mortality.
We presented the data as means or medians where appropriate using descriptive statistics. We assessed differences in demographic data and secondary measures between patient groups using χ2 analysis and Student t tests for categoric and scale data, respectively. An α significance level of 0.05 was set a priori. We performed all statistical testing using Stata/IC version 15.0 (StataCorp.).
Results
Thirty-five severely injured patients (31 [89%] male, median age 46 yr, median ISS score 30, blunt mechanism in 26 cases [74%]) underwent a hybrid intervention in the RAPTOR suite during the study period. Twelve patients (34%) received a truly simultaneous procedure, and 23 (66%) underwent a rapid serial procedure. There was no significant difference between the truly simultaneous and rapid serial groups in median age (49 yr v. 45 yr, p = 0.4), sex distribution (male sex 11 [92%] v. 20 [87%], p = 0.7), mechanism (blunt 11 [92%] v. 15 [65%], p = 0.09) or injury severity (median ISS score 34 v. 26, p = 0.2) (Table 1). However, differences were noted between the 2 groups in patient presentation (hemodynamic instability 11 [92%] v. 13 [56%], p = 0.03), requirement for massive transfusions (8 [67%] v. 7 [30%], p = 0.04), abdominal Abbreviated Injury Score score (median 5 v. 4, p = 0.01) and requirement for damage-control procedures (10 [83%] v. 12 [52%], p = 0.03).
Patient, injury, intervention and outcome demographic characteristics by type of RAPTOR intervention
Eight patients (67%) in the truly simultaneous cohort underwent a combination of open surgery, and either percutaneous embolization (6 patients) or endovascular stenting (2 patients) (Table 1). The liver and pelvis were the most common targets for embolization. Similar trends were identified in the rapid serial cohort. Resuscitative endovascular balloon occlusion of the aorta was performed in 2 patients with severe pelvic fractures in the rapid serial group.
Overall, 19 patients (54%) received a computed tomography (CT) imaging series before their procedure in the RAPTOR suite. The rate of preprocedural CT was lower in the truly simultaneous group than in the rapid serial group (3 [25%] v. 16 [70%], p = 0.01) (Table 1). This aligns well with more rapid care, as also evidenced by a shorter interval between patient presentation to the trauma bay and subsequent arrival at the RAPTOR suite (mean 31 min v. 59 min, p = 0.02).
Among open procedures, patients underwent laparotomy (30 [86%]), thoracotomy (6 [17%]) and/or an extremity vascular procedure (3 [9%]). Among percutaneous procedures, embolization (19 [54%]), endovascular aortic stent placement (5 [14%]) and diagnostic angiography alone (11 [31%]) were performed (Table 1). Target anatomy for embolization included the liver (11 [58%]), and bilateral or unilateral internal iliac arteries and their branches (8 [42%]). Target anatomy for isolated diagnostic angiography (i.e., open procedure + percutaneous diagnostic angiography alone) included the aorta (7 [64%]) and/or iliac arteries (4 [36%]).
The overall median length of hospital stay was 15 days. The majority of patients (19 [54%]) were discharged directly home. Discharge disposition was similar between the simultaneous and serial groups. The mortality rate was 14% (Table 1). The overall cause of death was uncontrolled hemorrhage in 4 patients (80%) and catastrophic brain injury in 1 (20%).
All patients benefitted from the efficiencies of a single environment with the capability to complete both of their required procedures in rapid format. This was particularly evident among patients in the truly simultaneous group. On detailed panel review, 11 patients (92%) in this group clearly benefitted from truly simultaneous procedures in the RAPTOR suite.
Optimal procedural set-up and human flow factors for truly simultaneous RAPTOR interventions
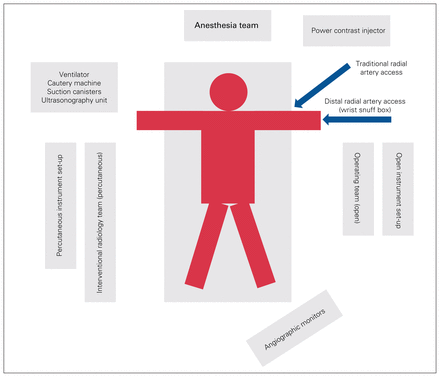
After debriefing of the truly simultaneous cases, we concluded that the optimal RAPTOR operating environment should include the following factors (Figure 1):
Patient and equipment positioning within the RAPTOR (resuscitation with angiography, percutaneous techniques and operative repair) suite.
Standard hybrid operating room components (e.g., carbon fibre table, fixed angiographic capabilities)
Preparation of 2 full instrument set-ups (open and percutaneous) at the beginning of the case
Two scrub nurses, 2 circulating nurses and 1 radiology technician
Positioning of the surgical team on the left of the patient, with angiography monitors near the foot of the bed (this allows rapid access to the left thorax if needed); positioning of the percutaneous team on the right of the patient (this allows direct visualization of the monitors)
Cautery machine, suction device(s), ventilator and ultrasonography unit, each positioned on the right of the patient above the arm board
Power contrast injector on the left of the patient
Early patient arterial access (radial or femoral) (for both monitoring and therapeutic interventions)
Standard patient trauma skin preparation (rapidly, from the angle of the mandible to the ankles)
Strategizing groin (or alternative) arterial access in patients who require pelvic binders
All team members (anesthesia, nursing, and percutaneous and surgical teams) should wear lead aprons/garments and eye protection
Closed-loop communication in a quiet and efficient working environment is critical, especially in the absence of headsets for team leaders
If truly simultaneous surgical and endovascular procedures are engaged, radiation protection for the entire health care team, with a focus on the surgeon, is essential. All percutaneous endovascular interventions require fluoroscopy. For example, if a patient with a severe liver injury requires rapid and sustained manual packing, as well as arterial embolization, the surgeons’ hands are at risk of being irradiated if left in place within the abdomen. The simultaneous treatment of a patient whose condition is unstable therefore works best when separate body areas are being treated at the same time. For example, in a patient with a high-grade splenic injury requiring splenectomy as well as active hemorrhage within the pelvis, the surgical team can remove the spleen while the interventional radiology team embolizes the pelvic arteries. Another optimized simultaneous example is embolization of a bleeding thoracic artery or aortic stent grafting while the surgical team works within the abdomen or pelvis. Safe radiation practices require the surgeon to remove his or her hands out of the fluoroscopic field. Another factor to consider in ensuring optimal surgical intervention is the physical positioning of the image intensifier (C-arm) itself. This will depend on the set-up of the specific environment but should be considered before initiating the procedure.
Regarding percutaneous access, left radial access is our preferred site for percutaneous body procedures, as it precludes crossing the aortic arch (as in right radial access) and, in our experience, lessens the chance of a stroke, especially in patients with trauma, in whom anticoagulation may be an issue. Left-sided radial access involves some arm/wrist placement considerations to facilitate good ergonomics and proper visualization of the abdomen and pelvis. Once the left arm is completely prepped in a sterile manner, it can then be bent 90° at the elbow (i.e., the left wrist is positioned close to the right hip). Radial access can then be performed in a conventional manner (i.e., via a traditional arterial line in any location along the artery) or, alternatively, more distally within the left wrist snuff box (which is more comfortable for patients who are awake). This position described above allows excellent visualization of the upper solid organs (spleen, liver, kidneys). For visualization of the pelvis, the left wrist is moved superiorly to the upper abdomen. Both positions allow the operator to work on the “normal,” right side of the patient (and therefore the surgical team on the left side if necessary). This can also be achieved with the abdomen exposed and the arm/wrist moved as needed. Although it is possible for the percutaneous team to work from the left side of the patient, this alignment becomes much more ergonomically challenging owing to equipment issues such as C-arm and screen positioning.
Discussion
The terminology used to describe truly hybrid operating environments remains heterogeneous. In this study, we have proposed and used 2 distinct definitions (Box 1). Simultaneous (synonymous with concurrent and synchronous) RAPTOR procedures refer to scenarios in which 2 separate teams (surgical and percutaneous, including associated nursing teams) are both scrubbed and operating at the same time. Rapid serial RAPTOR interventions refer to scenarios in which 1 team performs its procedural component first, followed by a second team (i.e., once the first team has stopped). This scenario may repeat itself again depending on the complexity and demands of the given patient’s injuries. Confusion within this terminology is not unique to injury care: in cases of synchronous resections for colorectal primary cancer and liver metastases, for example, the liver and colorectal surgical teams do not operate “simultaneously.”13 Instead, these teams perform rapid serial resections and reconstructions as necessary.
RAPTOR (resuscitation with angiography, percutaneous techniques and operative repair) terminology
Concurrent: synonymous with “simultaneous” and “synchronous”
Serial: 1 team performs its procedural component first, followed rapidly by a second team
Simultaneous: 2 separate teams (surgical and percutaneous) are both scrubbed and operating at precisely the same time
Synchronous: synonymous with “simultaneous” and “concurrent”
In this prospective audit and comparison of patients who required truly simultaneous versus rapid serial procedures to arrest ongoing hemorrhage, patients who required care from the surgical and percutaneous teams at the same time were more severely ill than those who required serial interventions. A higher proportion of the former were hemodynamically unstable, required damage-control procedures, received massive transfusions and received the benefit of preoperative CT, and they were transferred from the ambulance to the RAPTOR suite more rapidly. Despite these differences, the mortality rate among this patient cohort was similar to that among patients who underwent serial interventions (17% v. 13%). Length of stay was also similar between the 2 groups. These observations suggest that the trauma surgeons were able to identify more critically ill patients with ongoing hemorrhage and expedite their care to the RAPTOR suite. In the context of previous publications confirming both improved survival and more efficient care in the RAPTOR suite for patients with ongoing hemorrhage compared to the pre-RAPTOR era,7,11 it should be underlined that it is of the utmost importance to eliminate any obstacles in the prehospital setting, trauma bay/emergency department, and/or patient transfer process that slow the ability of the trauma surgeon to make an initial disposition decision for every bleeding patient.14
More than half (54%) of all patients underwent a CT scan before their hybrid operative intervention. This fits well with previous publications from both our centre,7,11 reinforcing the evolving use of rapid, close-proximity cross-sectional imaging before the intervention, as necessary, even in even the most critically ill patients. However, only 25% of our truly simultaneous cohort underwent preprocedural CT. This reflects the more critical nature of this group and therefore our unwillingness to introduce any avoidable delays in patient care pathways between the emergency department and the RAPTOR suite. It should be noted, however, that the specific location of the CT scanner within a given hospital will alter both availability and utility. Although low-fidelity cross-sectional images (e.g., basic CT of the brain) can be acquired in the RAPTOR suite, the formal trauma CT scanner in our centre is located immediately adjacent to the emergency department (i.e., on a separate floor from the RAPTOR suite).
On panel review, it was evident that 92% of the patients treated in a truly simultaneous manner clearly benefitted from a concurrent hybrid procedure. This is not overly surprising, given the mix of cases in this group (stenting of free rupture and bleeding aortas, active hemorrhage from pelvic arterial vessels in the context of hemodynamically unstable patients with pelvic fractures, and active hemorrhage from high-grade liver trauma).
A commentary regarding the importance of moving patients as rapidly as possible from the moment of injury through to the arrest of ongoing hemorrhage is also warranted. Review of the data from our institution showed that the interval from arrival at the trauma bay to procedural intervention decreased from 212 minutes when procedures were performed in an angiography suite, to 148 minutes in an angiography suite with quality improvements, to 101 minutes in an operating room, to 90 minutes in an operating room with quality improvements, to 82 minutes when the RAPTOR suite became available, to 31 minutes in the RAPTOR suite for the most critically ill patients requiring truly simultaneous procedures.7,11,15 This decrease is a direct result of the ability of the operating room staff and surgeon to mobilize effective resources and destination access at a much greater speed than was possible within an isolated angiography suite.7,11 It also reflects the unwavering commitment of trauma surgeons to be immediately present on patient arrival and move critically injured patients rapidly through potential delays associated with both prehospital and emergency department obstacles that delay definitive hemorrhage control. At the Foothills Medical Centre, a dedicated interventional radiology team is available at all times. This team consists of a staff interventional radiologist, technologist and specialized nurse. In cases of trauma activation, the trauma surgeon contacts the interventional radiologist directly, who then activates the interventional radiology team. During daytime hours, the expectation is arrival in the RAPTOR suite within 10 minutes; after hours, this interval extends to 25 minutes. Given the close working relationship between the 2 teams, there have been no coordination or sequencing issues to date.
The specific technical set-up for both operative and percutaneous interventions will vary across institutions and clinicians.11,12 There are, however, critical factors that must be considered in an environment where 2 separate teams with unique equipment and operating requirements will be working simultaneously. In addition to the recommendations listed in the Results section, these factors include adequate nuanced communication between the trauma surgeon and the percutaneous team (typically interventional radiologist); a rapid preprocedural briefing for all team members of the planned interventional and care sequence; the ability to rapidly obtain/set up both open and percutaneous equipment, with adequate patient access points for both teams; a quiet working environment that facilitates communication among the anesthesiologist and the surgical and percutaneous teams; and a clear division of space around the patient to facilitate simultaneous procedures (which must be flexible when required).
Although this article is not focused on training paradigms, the collegiality, camaraderie and bidirectional learning between interventional radiologists and trauma surgeons should ideally extend beyond the faculty level. More specifically, the training pathway of fellow trainees (both interventional radiology and surgery) must be considered. Although there has yet to be a formal requirement at the fellowship level, we recommend that our trauma surgery fellows complete rotations in interventional radiology, and vice versa. This provides advanced education in vascular access, wire skills, balloon and embolization “tricks,” and access closure devices. The opportunity to acquire technical nuances from our high-volume interventional radiology colleagues cannot be overstated. The duration of these rotations is tailored to the needs of the learners: if they will be responsible for percutaneous procedures in their subsequent faculty position, longer rotations are recommended. It should also be noted that, although the fidelity and availability of simulation environments could be greatly improved, regular team-based simulation scenarios can be extremely helpful in the maintenance of both hard skills (e.g., instrument set-up and hybrid environment arrangement) and softer components (e.g., closed-loop clear communication, noise control, quality improvement).
Limitations
The main limitations of this study include the fact that it was not a randomized trial, and therefore differences between groups are susceptible to bias, and that a formal economic analysis was not possible owing to the inability to track detailed patient-specific costs in the Canadian health care system. In addition, in a small sample of 35 patients, statistical comparisons are subject to potential instability: whether in a small randomized trial or a retrospective review, when a limited number of patients are hypothetically moved from one numerator to the other comparator, statistical conclusions can be altered substantially. Although the truly simultaneous and rapid serial groups differed significantly across several variables (including the Abbreviated Injury Scale score, use of damage-control procedures, massive transfusion requirement and hemodynamic instability), differences in additional variables such as mechanism of injury and ISS were also reasonably close to being significant (p = 0.09 and 0.2, respectively).
Conclusion
Truly simultaneous open and percutaneous procedures to stop ongoing hemorrhage were unique in both patient and procedural details. For the most severely injured patients, the provision of truly simultaneous modalities is necessary to achieve clinical outcomes equivalent to those of less ill patients.
Footnotes
Presented at the virtual 79th Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care Surgery, Sept. 8–18, 2020, Kona, Hawaii
Competing interests: Chad Ball is a coeditor-in-chief for CJS; he was not involved in the editorial decision-making process for this article. Andrew Kirkpatrick has consulted for ZOLL Medical Corporation, Acelity (3M/KCI), CSL Behring, Innovative Trauma Care, SAM Medical and The Statesman Group of Companies. He has been loaned a computer, defibrillator and ultrasonography unit to assist with consulting for ZOLL Medical Corporation. He is principal investigator on a randomized trial partially supported by Acelity and is a member of the Executive Committee of the Abdominal Compartment Society. No other competing interests were declared.
Contributors: C. Ball and T. Clements designed the study. C. Ball, T. Clements and J. Wong acquired the data, which A. Kirkpatrick and J. Wong analyzed. C. Ball wrote the manuscript, which A. Kirkpatrick, J. Wong and T. Clements critically revised. All authors gave final approval of the article to be published.
- Accepted January 7, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/