Abstract
Background: There is a paucity of literature exploring the role of bariatric surgery in class 1 obesity. We evaluated the 5-year outcomes after bariatric surgery in patients with class 1 obesity, assessing weight loss, resolution/reduction of obesity-related comorbidities, morbidity and mortality.
Methods: We performed a single-centre retrospective analysis of patients who underwent bariatric surgery (laparoscopic sleeve gastrectomy [LSG] or laparoscopic Roux-en-Y gastric bypass [LRYGB)]) for class 1 obesity (body mass index [BMI] 30.0–34.9) between January 2012 and February 2019.
Results: Thirty-seven patients (35 [95%] female, mean age 44.5 yr [standard error (SE) 11.3 yr], mean preoperative BMI 33.1) were included, of whom 32 underwent LSG and 5 underwent LRYGB. Thirty-five patients were followed for 5 years post-operatively, achieving a mean BMI of 25.6 (SE 1.2) and excess weight loss of 89.4% (SE 15.1%). Remission of hypertension was achieved in 5 of 12 patients (42%), and remission of dyslipidemia was achieved in 7 of 11 patients (64%). Of the 11 patients with diabetes, 7 underwent LSG and 4, LRYGB. At 5 years postoperatively, the mean glycosylated hemoglobin concentration was 6.3%. Four patients in the LSG group developed de novo reflux, 1 patient required conversion to LRYGB, and 1 patient with sleeve stenosis required endoscopic dilatation. There were no deaths in either patient group.
Conclusion: At our centre, bariatric surgery for class 1 obesity was safe and had long-term efficacy, with remission or reduction of related comorbidities. Prospective controlled trials are required to confirm these results.
Despite national awareness and multiple campaigns highlighting the importance of maintaining an ideal body weight, the proportion of Canadians with a body mass index (BMI) greater than 30 continues to increase.1–4 Since bariatric surgery has proven to be the most effective treatment modality for obesity,5 indications to perform bariatric surgery in patients with class 1 obesity should be based on comorbidities and the metabolic and psychologic burden of obesity, and then on BMI.2,5 This approach has been recommended by the International Federation for the Surgery of Obesity and Metabolic Disorders.2
Despite documentation of long-term failure of nonsurgical treatment modalities,5,6 the American Society for Metabolic and Bariatric Surgery released position statements in 2013 and 2018 regarding the lack of evidence supporting the efficacy of bariatric surgery for class 1 obesity.5,7 However, surgery provides efficient long-term management, and, as such, this position has been challenged.8,9
The benefits and safety of bariatric surgery for class 1 obesity have been reported.10–15 However, data on long-term outcomes are lacking. Thus, we evaluated the 5-year outcomes after bariatric surgery in patients with class 1 obesity (BMI 30.0–34.9), assessing weight loss, resolution/reduction of obesity-related comorbidities, morbidity and mortality.
Methods
Setting and design
We conducted a retrospective chart review of patients with class 1 obesity in the Department of Bariatric Surgery at Sacré-Cœur Hospital, Montréal, Quebec, Canada, between January 2012 and February 2019. In our institution, since 2009, patients with class 1 obesity have been treated with adjustable gastric banding, gastric plication, sleeve gastrectomy or Roux-en-Y gastric bypass (RYGB). As adjustable gastric banding and gastric plication are no longer performed,16,17 we studied outcomes of those who underwent primary laparoscopic sleeve gastrectomy (LSG) or laparoscopic RYGB (LRYGB). The study methodology was in accordance with the Canadian Journal of Surgery protocol for consensus-based articles.18 Informed consent was obtained from participants. All procedures were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Surgical procedures
The bariatric surgical procedures and follow-up were performed by 1 of 4 surgeons (P.Y.G., R.P., R.D. or H.A.). Procedures were performed as outpatient surgery whenever possible.19 Laparoscopic sleeve gastrectomies were calibrated with a 36- or 40-French bougie, according to surgeon preference. For cases involving concurrent laparoscopic adjustable band removal, the surgeon made a decision intraoperatively regarding the safety and feasibility of a single-stage procedure, taking into account band erosion and severe adhesions. All LRYGB procedures were performed with an antecolic antegastric Roux configuration, with an alimentary limb and biliary limb of 100–120 cm and 60–80 cm, respectively.
Endoscopy was performed preoperatively in patients who reported symptoms of gastroesophageal reflux. The chosen procedure was tailored according to the endoscopic findings and patient comorbidities.
Patients were followed in the outpatient clinic 1 month, 6 months and 12 months postoperatively, then annually thereafter. They were telephoned by 1 of 2 supervised medical students partaking in research projects associated with their medical degree.
Data sources
All available baseline and follow-up data were extracted from our bariatric database and from the patients’ charts. The investigators were blinded to patient details (BMI, type and date of intervention, and preoperative comorbidities). We extracted early postoperative morbidity (including intra-abdominal hematoma, abscess formation and leaks) and mortality. Development or exacerbation of gastroesophageal reflux and weight regain were also documented, with revision surgery rates recorded.
We also extracted the effects of surgery on metabolic comorbidities. We defined reduction of hypertension as a decrease in the dosage or number of antihypertensive medications required, and remission as blood pressure less than 120/80 mm Hg in the absence of antihypertensive medication.20 Blood pressure was measured by the family physician at early postoperative follow-up and routinely during surgical office follow-up. We defined improvement of dyslipidemia as a decrease in the dosage or number of lipid-lowering agents, with equivalent control of dyslipidemia or improved control of lipid values on equivalent medication, and remission as normal lipid profile without medication. We defined complete remission of diabetes as a glycosylated hemoglobin (HbA1c) level less than 6% in the absence of pharmacologic therapy, and partial remission as an HbA1c level less than 6.5% in the absence of pharmacologic therapy.21 Glycemic control was defined as an HbA1c level less than 7% with or without diabetic medications, and glycemic improvement was considered when the patient did not achieve the ideal target but achieved a 1.5% reduction in HbA1c level.21 We used the Dossier santé Québec (https://www.quebec.ca/sante/vos-informations-de-sante/dossier-sante-quebec) to monitor the pharmacologic profile and laboratory results of all patients and determine whether resolution/remission of their comorbidities were constant during the follow-up period.
Statistical analysis
For analysis of BMI, excess weight loss (EWL) and total weight loss (TWL), we conducted 2 separate repeated-measures mixed linear models using PROC MIXED in SAS (SAS Institute), with time as the independent variable. Baseline age, sex, baseline BMI, surgery type, date of surgery and method of follow-up (in person or via telephone) were included as covariates in both models. To mitigate the impact of any missing data, these analyses used all available data to provide estimates for each year of follow-up.
Results
A total of 37 patients (35 [95%] female, mean age 44.5 yr [standard error (SE) 11.3 yr], mean preoperative BMI 33.1 [range 30.1–34.9]) were included. The patients’ characteristics are shown in Table 1. Six (16%) of the 37 patients had previously undergone laparoscopic adjustable gastric banding for management of obesity. Indications for removal of the band included dysphagia, slippage and patient preference. Two patients underwent a single-stage procedure, and 4 patients underwent a 2-stage procedure to create a sleeve for persisting class 1 obesity. Of the 37 patients, 32 (86%) underwent LSG and 5 (14%) underwent LRYGB. The latter was indicated in 4 patients with poor glycemic control and large insulin requirements, and 1 patient with severe reflux disease nonresponsive to medical therapy. Eighteen (56%) of the LSG procedures were performed as day surgery.
Demographic and clinical characteristics of patients with class 1 obesity* who underwent bariatric surgery
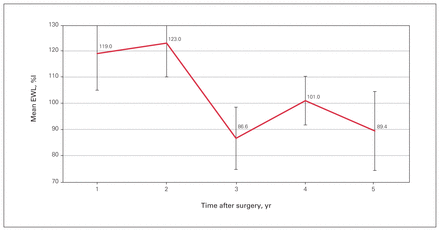
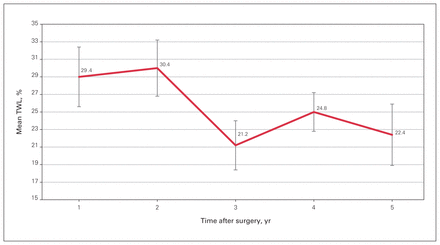
Thirty-five patients (95%) were followed for 5 years postoperatively. The average number of follow-up measures per patient was 2 (range 1–5). The lowest BMI, 19.7, was achieved in a 63-year-old patient at 1 year after LRYGB; otherwise, no other patient achieved a BMI less than 20. The mean BMI 3 and 5 years postoperatively was 26.1 (SE 1.0) and 25.6 (SE 1.2), respectively, with no statistically significant variations across time (F = 1.08, p = 0.4) (Figure 1). The corresponding values for mean EWL were 86.6% (SE 11.9%) and 89.4% (SE 15.1%), with no statistically significant variations across time (F = 1.01, p = 0.4) (Figure 2), and for mean TWL, 21.2% (SE 2.8%) and 22.4% (SE 3.5%), again with no statistically significant variations across time (F = 1.06, p = 0.4) (Figure 3).
Mean postoperative body mass index (BMI) in patients with class 1 obesity who underwent bariatric surgery.
Mean postoperative excess weight loss (EWL).
Mean postoperative total weight loss (TWL).
Comorbidity outcomes
The majority of patients (22 [59%]) had at least 1 obesity-related comorbidity (including high blood pressure, dyslipidemia, sleep apnea/hypopnea syndrome and diabetes). Of the 22 patients, 9 had 1 obesity-related comorbidity, 5 had 2 obesity-related comorbidities, and 8 had 3 or more obesity-related comorbidities. The effectiveness of surgery in relation to weight loss and reduction/remission of comorbidities is presented in Table 2. Eleven patients (30%) had type 2 diabetes, of whom 4 underwent LRYGB and 7 underwent LSG. The effectiveness of bariatric surgery on diabetes resolution is shown in Table 3.
Effectiveness of surgery in relation to comorbidity resolution, as indicated by reduction in or cessation of medications
Effectiveness of surgery in relation to diabetes resolution
At 5 years postoperatively, the mean HbA1c level was 6.3% (range 5.1%–7.9%), the mean BMI was 25.6 (SE 1.2), and the mean EWL was 89.4% (SE 15.1%).
Postoperative morbidity and mortality
In the LSG group, 1 patient (3%) experienced an intraabdominal hematoma, which was managed conservatively; this patient made an otherwise uneventful recovery. Four patients (12%) developed de novo reflux. In this subset, 1 patient developed reflux secondary to gastric stenosis, which required endoscopic dilatation at 18 months. One patient developed intractable reflux, with endoscopic findings of grade B esophagitis (Los Angeles Classification), associated with a 3 cm hiatal hernia and torsion of the sleeve. This required subsequent conversion to LRYGB and hiatal hernia repair 78 months postoperatively. In the remaining 2 patients, symptoms were controlled with the aid of long-term treatment with proton pump inhibitors; on subsequent endoscopy, no reflux-associated changes were seen.
In the LRYGB group, no cases of dumping, postprandial hypoglycemia or malnutrition developed over the 5-year period.
There were no deaths in either surgical group.
Discussion
Our results show that bariatric/metabolic surgery has long-term efficacy, with reduction or remission of related comorbidities, and is an effective treatment modality for patients with class 1 obesity. The American Society for Metabolic and Bariatric Surgery suggests that patients with a BMI of 30–35 with type 2 diabetes be strongly considered for bariatric surgery.5 Our study provides additional evidence to suggest lowering the initial BMI threshold to qualify patients to undergo metabolic surgery.
Maiz and colleagues11 reported their outcomes in a retrospective series of 1119 patients with class 1 obesity, of whom 283 underwent LRYGB and 836 had LSG. At 1 year, the patients achieved a mean BMI of 24.5 and mean EWL of 107.9%. However, that large series was limited by short follow-up. In our study, at 5 years postoperatively, the corresponding values were 25.6 and 89.4%.
Maiz and colleagues11 reported high rates of resolution of hypertension, diabetes and dyslipidemia (58%, 54% and 54%, respectively). Our findings were similar, with cessation of medications recorded for 42% of patients with baseline hypertension, 64% of patients with hyperlipidemia and 54% of patients with diabetes.
Buchwald and colleagues22 reported that 48% of patients who underwent laparoscopic adjustable gastric banding, 84% of those who had gastric bypass and 98% of those who had biliopancreatic diversion had significant resolution of their poor glycemic control. Their meta-analysis showed that normoglycemia can be achieved within days after gastric bypass, before substantial weight loss, and that 87% of patients with diabetes had improvement or remission of type 2 diabetes after bariatric/metabolic surgery.23 In a small study, de Sa and colleagues24 studied 27 patients with class 1 obesity who underwent RYGB (with alimentary and biliary limbs of 150 cm and 100 cm, respectively) and reported mean BMI and HbA1c levels of 25 and 6%, respectively at 20 months. Resolution of type 2 diabetes was achieved in 48% of patients, and glycemic control without medication was achieved in 74%. However, the follow-up period was relatively short. In a study similar to ours, Ferraz and colleagues25 followed a small cohort of patients with class 1 obesity who underwent LRYGB for 6 years; complete remission of diabetes and glycemic control were achieved in 16.7% and 25% of patients, respectively. Abbatini and colleagues26 examined 9 patients with class 1 obesity who underwent LSG and found that, at 1 year, 89% of patients had remission of their type 2 diabetes. Most patients did not require insulin therapy preoperatively and had a duration of diabetes of less than 10 years. Our results are in agreement with these studies. The similar outcomes across studies highlight the reproducibility of the results, and the importance of preventive surgery and early intervention to avert the serious vascular sequelae associated with long-term poor glycemic control.
Our results suggest that LSG and LRYGB are safe treatment modalities for class 1 obesity, with an acceptably low postoperative morbidity rate of 2.7% and no deaths. Varban and colleagues10 observed morbidity and mortality rates of 3.4% and 0.44%, respectively, among patients who underwent sleeve gastrectomy. In their study of patients with class 1 obesity who underwent bariatric surgery, Feng and colleagues12 reported 30-day reoperation rates of 1.01% among 6234 patients who underwent LSG and 2.94% among 1838 patients who underwent RYGB.
As well, with the emerging endoscopic armamentarium, surgical intervention for management of complications is decreasing. Gamme and colleagues13 reported complication rates of 3.9% and 3.5% for patients with class 1 obesity and those with class 2 or greater obesity, respectively. Gastroesophageal reflux disease is an obesity-related comorbidity but is also a complication of bariatric surgery. In our study, the rate of revisional surgery for intractable reflux after LSG was 2.7%. There was a high prevalence of reflux preoperatively in this group,27,28 with 21% of patients experiencing symptomatic reflux, which was well managed with proton pump inhibitors. Given the traditionally higher complication rates associated with LRYGB,11,12 concerns about this procedure in patients with class 1 obesity and diabetes who are insulin dependent may be a consideration. Feng and colleagues12 compared morbidity rates among patients with diabetes who underwent LSG or LRYGB and found a significantly lower rate among patients who had oral hypoglycemic therapy (3.4% in the LSG group v. 6.8% in the LRYGB group, p < 0.001); however, there was no difference among patients who required preoperative insulin therapy (5.4% v. 8.1%, respectively, p = 0.09). A window of opportunity exists for early surgical intervention, as opposed to waiting for the highly probable weight regain and higher BMI values.
Body mass index alone is a poor index of adiposity and a poor marker of the health risks associated with obesity.2 Psychologic and clinical factors such as fat distribution, cardiovascular risk, presence of comorbidities and signs of organ damage should be taken into consideration when deciding whether to operate in patients with class 1 obesity, should they be unable to sustain adequate weight loss after a reasonable period of nonsurgical therapy.29 The benefits need to be balanced against the potential for surgical complications and the costs of bariatric surgery.30 Prospective controlled trials are required to further establish the true long-term safety and efficacy of bariatric/ metabolic surgery such as LSG and LRYGB in this population. However, this might be difficult for several reasons: in many countries, surgery in class 1 obesity is not financially supported by health insurance, caregivers still believe in lifestyle modification interventions for this class of patients, and long-term follow-up is challenging.31,32
Limitations
Limitations include the study’s retrospective design and the small cohort.
Conclusion
This study showed that bariatric/metabolic surgery is an effective treatment modality up to 5 years for patients with class 1 obesity, with remission or reduction of obesity-related comorbidities. A larger sample is needed to confirm the conclusions about safety. Marked improvement in glycemic control was seen in patients with class 1 obesity and diabetes. The results suggest a need to extend the criteria for bariatric/ metabolic surgery to lower BMI values. Once the surgical indication is accepted, the type of bariatric procedure must be tailored to the patient’s needs (weight loss, easing of psychologic burden and/or resolution of metabolic comorbidities), with special emphasis on diabetes and its severity.
Acknowledgement
The authors thank medical students Ahmed Amine Alaoui and Ayden Aboud, who helped with data collection and follow-up telephone calls.
Footnotes
Competing interests: None declared.
Contributors: A.-S. Studer, M. Magdy, R. Pescarus and H. Atlas designed the study. R. Denis acquired the data, which S. Bacon and P. Garneau analyzed. A.-S. Studer, M. Magdy and H. Atlas wrote the manuscript, which S. Bacon, R. Denis, R. Pescarus and P. Garneau critically revised. All authors gave final approval of the article to be published.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Simon Bacon is supported by a Canadian Institutes of Health Research Strategy for Patient-Oriented Research Mentorship Chair (SMC-151518) and a Fonds de recherche du Québec – Santé Chair (251618).
- Accepted January 9, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/