Abstract
Background: In a large nationwide mass vaccination setting, the SARS-CoV-2 vaccine was recently linked to myocarditis, lymphadenopathy, herpes zoster infection and appendicitis. We aimed to examine the characteristics and management of SARS-CoV-2 vaccine-related acute appendicitis.
Methods: We performed a retrospective cohort study in a large tertiary medical centre in Israel. All patients presenting with acute appendicitis within 21 days of receiving their SARS-CoV-2 vaccination (PCVAA group) were compared with patients who presented with acute appendicitis not related to the vaccination (N-PCVAA group).
Results: We reviewed the records of 421 patients with acute appendicitis from December 2020 to September 2021; 38 (9%) patients presented with acute appendicitis within 21 days of receiving their SARS-CoV-2 vaccination. Patients in the PCVAA group were older than those in the N-PCVAA group (mean 41 ± 19 yr v. 33 ± 15 yr, respectively, p = 0.008), with male predominance. More patients were managed nonsurgically during the pandemic than before the pandemic (24% v. 18%, p = 0.03).
Conclusion: With the exception of older age, the clinical characteristics of patients presenting with acute appendicitis within 21 days of receiving the SARS-CoV-2 vaccination did not differ from those of patients who presented with acute appendicitis not related to the vaccination. This finding suggests that vaccine-related acute appendicitis is similar to “classic” acute appendicitis.
The first cases of SARS-CoV-2 were reported in December 2019, in Wuhan, China, and the virus then spread worldwide over the next few months. More than 112 million cases of COVID-19 occurred within the first year with nearly 2.5 million deaths worldwide. Several SARS-CoV-2 vaccines have since been developed with the BNT162b2 mRNA vaccine (Pfizer-BioNTech) being the main one used in Israel.
In December 2020, before the beginning of the SARS-CoV-2 vaccination program, the United States Food and Drug Administration (FDA) Advisory Committee performed an evaluation regarding BNT162b2 mRNA vaccine safety and its adverse events. Acute appendicitis was reported as a serious adverse event that occurred in 8 participants in the vaccinated group, a higher rate than in the control group. Study investigators considered these cases to be unrelated to vaccination, and the FDA declared that there was no clear evidence that these findings were related to the vaccine.1 Lymphadenopathy, mostly in the axilla and neck, was observed in the initial phase 3 trials of both the mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) vaccines2,3 as an adverse effect of the vaccine and was later reported in further studies.4,5
In Israel, the vaccine campaign was started on Dec. 20, 2020, based on the 2-dose BNT162b2 course, spaced 21 days apart. The campaign first targeted health care workers and people older than 60 years and then quickly extended to the general population aged 16 years and older. By June 26, 2021, about 64% of eligible people in Israel had received at least 1 dose.6
Barda and colleagues7 analyzed the outcomes of 884 828 people who were vaccinated in Israel. Several potential adverse events associated with the vaccine were identified, including myocarditis, lymphadenopathy and acute appendicitis.7 The risk for acute appendicitis, the most common surgical condition with a lifetime incidence of 7%–8%, was reported to be elevated after SARS-CoV-2 vaccination (risk ratio 1.40; 95% confidence interval [CI] 1.02–2.01; risk difference 5.0 events per 100 000 people; 95% CI 0.3–9.9). Other studies highlighting the association between acute appendicitis and SARS-CoV-2 vaccination have not shown a significant association.8
With lymphoid tissue proliferation established as an adverse effect of the vaccine, a potential mechanism for acute appendicitis following the SARS-CoV-2 vaccination could be lymphoid activation and appendiceal obstruction, creating a different disease entity than “classic” acute appendicitis. To test this hypothesis, we compared the clinical and epidemiological characteristics of patients who presented with acute appendicitis within 21 days of receiving their SARS-CoV-2 vaccination (PCVAA group) with patients who had acute appendicitis not related to the SARS-CoV-2 vaccination (N-PCVAA group). In addition, we compared these 2 groups to a historical cohort of patients who were admitted with acute appendicitis before the onset of the pandemic.
Methods
Setting and ethical approval
Soroka University Medical Center (SUMC) is a tertiary 1040-bed centre serving more than 750000 inhabitants, located in the city of Beer Sheva in southern Israel. The SUMC institutional ethics committee reviewed and approved this study (approval no. 0268–21-SOR).
Study participants and exposure
We retrospectively reviewed the records of all patients with acute appendicitis aged 16 years and older who had been admitted to our centre from Dec. 20, 2020 (the beginning of the vaccination campaign in Israel), to Sept. 24, 2021. The diagnoses were based on clinical and radiological methods, including ultrasonography, computed tomography and magnetic resonance imaging. In addition, we reviewed the records of patients with acute appendicitis from December 2018 to September 2019, herein termed pre-COVID-19 era acute appendicitis. Only mRNA vaccines were administered in Israel in the study period, and almost all were BNT162b2.
Data extraction and analysis
Data were extracted from Clalit Health Services using its health care data sharing platform MDClone ADAMS.
According to previously published data, we assumed that the vaccine effect on acute appendicitis incidence was significant during the first 21 days after dose administration.7 Hence, the PCVAA group included patients who presented with acute appendicitis within 21 days of each dose of the SARS-CoV-2 vaccine, for a total of 42 days. The N-PCVAA group was divided into 3 subgroups: patients with SARS-CoV-2 infection before diagnosis of acute appendicitis, patients who received the SARS-CoV-2 vaccination more than 21 days before diagnosis of acute appendicitis and patients with acute appendicitis who were not vaccinated and without SARS-CoV-2 infection.
The data included demographic characteristics, relevant medical background, including vaccination status and previous SARS-CoV-2 infection; current acute appendicitis disease, including laboratory and radiological tests; surgical or medical management and course; and short-term (30 d) complications.
Statistical analysis
Analysis was performed with the use of R software, version 4.0.5 (R Foundation for Statistical Computing). Categorical data are presented as numbers and percentages, with differences assessed using the Pearson χ2 test for general association. Numerical results are presented as means ± standard deviation (SD). We used the Student t test for comparison of normally distributed data and the Mann–Whitney U, Wilcoxon rank-sum and Kruskal–Wallis tests for comparison of non-normally distributed data. All analyses were 2-sided and a p value of less than 0.05 was considered to be statistically significant.
Results
We reviewed the records of 421 patients with acute appendicitis who were admitted to our medical centre during December 2020–September 2021: 38 (9%) in the PCVAA group) and 383 (91%) in the N-PCVAA group (Table 1). In the N-PCVAA group, 14 had SARS-CoV-2 infection before their diagnosis of acute appendicitis, 136 received the SARS-CoV-2 vaccination more than 21 days before diagnosis of acute appendicitis and 233 were not vaccinated and had not been infected with SARS-CoV-2 (Table 2).
Comparison between the PCVAA group and the N-PCVAA group, during the pandemic
Comparison between the PCVAA group and the N-PCVAA subgroups of patients, during the pandemic
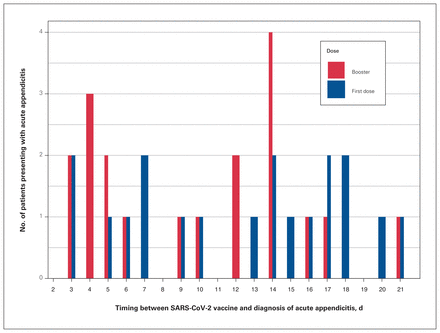
In the PCVAA group, 19 patients presented with acute appendicitis after their first dose of the vaccine and 19 patients after their second dose. The times from vaccination until diagnosis of acute appendicitis were homogeneously distributed (Figure 1).
Distribution (days) of acute appendicitis after exposure to the SARS-CoV-2 vaccination.
Patients in the PCVAA group were significantly older than those in the N-PCVAA group (mean 41 ± 19 yr v. 33 ± 15 yr, respectively, p = 0.008). However, the mean age of those in the N-PCVAA subgroup vaccinated more than 21 days before the diagnosis of acute appendicitis was similar to the age in the PCVAA group (41 ± 19 yr v. 38 ± 17 yr, respectively, p = 0.40).
During the pandemic, most patients who presented with acute appendicitis were male (239/421 [57%]). This predominance was more pronounced in the PCVAA group than in the N-PCVAA group but did not reach statistical significance (27/38 [71 %] v. 212/383 [55 %], p = 0.06).
No difference was found in the laboratory inflammatory indices between the PCVAA and N-PCVAA groups, including white blood cell count (12.0 ± 4.1 × 109/L v. 12.8 ± 4.3 × 109/L [normal: 4.5–11.0 × 109/L], respectively, p = 0.13) and C-reactive protein (5.43 ± 7.62 nmol/L v. 4.48 ± 5.90 nmol/L [normal: 0.76–28.5 nmol/L], respectively, p = 0.50). While levels of serum creatinine in patients in the PCVAA group were significantly higher than those of patients in the N-PCVAA group, this difference was not significant (76.91 ± 22.98 μmol/L v. 67.18 ± 17.68 μmol/L [normal: 53–106 μmol/L], respectively, p = 0.01).
The radiological reports of patients in the PCVAA group were reviewed. Except for 1, all patients presented with radiological signs of acute inflammation (wall thickening or periappendiceal fat stranding). Other signs, regularly seen in patients with acute appendicitis, were also found (5 with abdominal lymphadenopathy, 6 with appendiceal appendicolith and 7 with intra-abdominal fluid).
No difference was found in the rate of surgical compared with conservative management between the PCVAA and N-PCVAA groups (30/38 [79%] v. 292/383 [76%], respectively, p = 0.70). There was no significant difference in length of hospital stay (30 d), emergency department referrals and readmission to hospital.
A secondary analysis was performed, comparing the 464 patients with acute appendicitis during the corresponding pre-COVID-19 era (Table 3) to either the PCVAA or N-PCVAA groups. No differences in disease characteristics or treatment were found, except for a reduction in the rate of surgical management during SARS-CoV-2 outbreaks (82% v. 76%, respectively, p = 0.03).
Comparison between patients with acute appendicitis during the pandemic with acute appendicitis pre-COVID-19 era
Discussion
Acute appendicitis is the most common indication for abdominal emergency surgery worldwide. The peak incidence usually occurs in the second or third decade of life, with a slightly greater prevalence in males.9 The exact pathophysiology of acute appendicitis is unknown, but it is thought to occur most commonly due to obstruction of the appendiceal lumen and classically due to fecalith, lymphoid hyperplasia or tumour.10,11
We investigated the characteristics of patients in the PCVAA group and compared them to those of patients in the N-PCVAA group. The PCVAA group accounted for 9% of all patients with acute appendicitis during the study period. These patients were significantly older than those in the N-PCVAA group, with a mean age of 43 years, similar to that reported in the studies by Barda and colleagues7 and Dagan and colleagues,12 and in contrast to the known peak incidence of acute appendicitis, which is reported to be in late childhood and early adulthood.11 This is most likely associated with the older age of the vaccinated population, as vaccine rollout was gradual with vaccination of people aged 16 years and older beginning about 2 months after vaccination of people aged 50 years and older. This hypothesis is further supported by the subgroup analysis of patients excluded from the PCVAA group owing to a period of longer than 21 days between vaccination and acute appendicitis — a group that was similar in age to the patients in the PCVAA group.
The predominance of male sex was greater in the PCVAA group than in the N-PCVAA group (71% v. 55%, p = 0.06). Male predominance in acute appendicitis is known, but less significant than in our study.9 We do not have a plausible explanation for this difference; however, male predominance has also been reported for other vaccine-related adverse effects, such as myocarditis,13 and the underlying cause could be similar.
The appearance of acute appendicitis was distributed homogeneously after the first and second doses of the vaccine, with 19 cases after each dose (Figure 1). No previous reports on the effect of the number of doses on the prevalence of acute appendicitis have been reported. Homogeneity was also observed in the time between vaccination and diagnosis of acute appendicitis. These findings stand in contrast to those of Mitchell and Yue,8 which showed higher prevalence of acute appendicitis in the first days after SARS-CoV-2 vaccination. In addition, the incidence of myocarditis after SARS-CoV-2 vaccination was also reported to be higher in the study by Mevorach and colleagues in the first days after the second dose.13 We believe that the homogeneity of the distribution in our study results from the relatively small group of patients or can point to the fact that there is no causative correlation between vaccination and acute appendicitis.
A small but statistically significant increase in creatinine levels in the PCVAA group was found, but we do not believe this difference to be clinically significant. No differences were found in inflammatory laboratory markers between groups.
This study showed a reduction in the rate of surgical management of acute appendicitis during the COVID-19 pandemic compared with the pre-COVID-19 era. This trend was observed in other studies14 and in a metaanalysis of 14 studies. This is possibly the result of the general trend of nonoperative management for acute appendicitis in recent years,15 in conjunction with the reduction in health care resources during the pandemic that led the medical teams to pursue less burdensome solutions for common clinical situations.16
As periappendiceal lymphadenopathy and intraluminal appendiceal lymphatic tissue hyperplasia are related to acute appendicitis, and as lymphadenopathy is one of the adverse events associated with SARS-CoV-2 vaccination, one might expect to have found a higher incidence of lymphadenopathy in the imaging studies of patients in the PCVAA group in our study. Nevertheless, there were only 5 cases of lymphadenopathy reported in the imaging studies in this group. This could be explained by under-reporting of imaging studies or by a different mechanism leading to patients who presented with acute appendicitis within 21 days of receiving their SARS-CoV-2 vaccination.
Limitations
One of the limitations of our study is the lack of pathological results reported. Therefore, we cannot draw firm conclusions about the relation between lymphadenopathy and acute appendicitis after SARS-CoV-2 vaccination. A possible explanation for the lack of any substantial difference between the 2 groups or of a strict timeline for acute appendicitis occurrence after vaccination could be that this association is purely coincidental and that there is no causal relation between SARS-CoV-2 vaccination and acute appendicitis.
Conclusion
The occurrence of acute appendicitis might be increased after SARS-CoV-2 vaccination, possibly owing to lymph node activation. Our study shows that the characteristics and outcomes of patients with acute appendicitis after the SARS-CoV-2 vaccination are not different from those of patients presenting with acute appendicitis unrelated to vaccination. We believe that a study of histological and pathological factors of the resected appendix may shed more light on the pathophysiology of acute appendicitis after SARS-CoV-2 vaccination. However, the lack of clinically significant differences between our study groups could also stem from a lack of association between the SARS-CoV-2 vaccination and acute appendicitis, with our PCVAA group being an accidental cohort defined by vaccination timing rather than a pathophysiological process related to vaccination.
Footnotes
Competing interests: None declared.
Contributors: I. Kukeev, E. Quint and G. Sebbag designed the study. I. Kukeev acquired the data, which D. Czeiger, O. Dukhno, D. Grupel, G. Ohad, I. Hazan and A. Osyntsov analyzed. O. Dukhno, G. Ohad, I. Kukeev, E. Quint wrote the article, which D. Czeiger, D. Grupel, I. Hazan, A. Osyntsov and G. Sebbag reviewed. All authors approved the final version for publication.
- Accepted February 27, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use) and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/