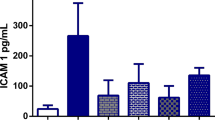
Abstract
This study looks at the role of xanthine oxidase (XO) in ischemia/reperfusion (I/R) induced intestinal mucosal damage using normal and xanthine oxidase deficient rats. Tungstate feeding for 3 days depleted the intestinal mucosal XO by 80%. A ligated loop of the rat small intestine (both normal and XO-deficient) was subjected to 1 h of total ischemia followed by 5 min revascularisation. The ensuing mucosal damage was assessed by biochemical and histological studies. Ischemia or I/R increased the XO levels in normal rats without any change in XO-deficient rats. Myeloperoxidase (a neutrophil marker) level was increased in both group of rats but it was comparatively higher in the XO-deficient rats. Accumulation of peroxidation products such as malondialdehyde, conjugated diene and increased production of hydroxyl radicals by microsomes were seen after ischemia and I/R and were similar in normal and XO-deficient rats. Studies on other parameters of peroxidation showed a decrease in polyunsaturated fatty acids and alpha-tocopherol, an increase in cysteine and cystine levels after I/R and were similar in both normal and XO-deficient rats. Histological results indicated gross morphological changes in the intestinal mucosa due to ischemia and I/R, and the damage was more severe in XO-deficient rats. These observations suggest that oxygen-derived free radicals are involved in the intestinal mucosal damage during I/R and infiltrated neutrophils rather than XO may be the primary source of free radicals under these conditions.
Similar content being viewed by others
References
Granger DN, McCord JM, Parks DA, Hallwarth ME: Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterol 90: 80–84, 1985
Jolly SR, Kane WJ, Baille MB, Abrams GD, Lucchesi BR: Canine myocardial reperfusion injury: its reduction by the combined administration of SODase and catalase. Circ Res 54: 277–285, 1984
Parks DA, Bulkley GB, Granger DN: Role of oxygen-derived free radicals in digestive tract diseases. Surgery 94: 415–422, 1983
Parks DA, Granger DN: Ischemia induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Amer J Physiol 245: G285-G289, 1983
Bus JS, Gibson JE: Lipid peroxidation and its role in toxicology. Rev Biochem Tox 1: 125–149, 1979
McCord JM: Superoxide, SODase and oxygen toxicity. Rev Biochem Tox 1: 109–123, 1979
Parks DA, Shah AK, Granger DN: Oxygen radicals: effects on intestinal vascular permeability. Amer J Physiol 247: G167-G170, 1984
Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS: Role of XO inhibitor as free radical scavenger: a novel mechanism of action of allopurinol & oxypurinol in myocardial salvage. Biochem Biophys Res Commun 148: 314–319, 1987
Vasko KA, DeWall RA, Riley AM: Effect of allopurinol in renal ischemia. Surgery 71: 787–790, 1972
Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JMC: Allopurinol & Oxypurinol are hydroxyl scavengers. FEBS Lett 213: 23–28, 1987
Zimmerman BJ, Parks DA, Grisham MB, Granger DN: Allopurinol does not enhance antioxidant properties of extracellular fluid. Amer J Physiol 255: H202-H206, 1988
Peterson DA, Kelly B, Gerrard JM: Allopurinol can act as an electron transfer agent. Is this relevant during reperfusion injury? Biochem Biophys Res Commun 137: 76–79, 1986
Topham RW, Walker WC, Callish MP: Liver XDH and iron mobilization. Biochem Biophys Res Commun 109: 1240–1246, 1982
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Slater TF, Sawyer BC: The stimulatory effect of carbon tetrachloride and other halogeno alkanes on peroxidative reactives of rat liver fractionsin vitro. Biochem J 123: 815–824, 1971
Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959
Kakielka E, Cederbaum AI: NADH dependant microsomal interaction with ferric complexes and production of reactive oxygen intermediates. Arch Biochem Biophys 275: 540–550, 1989
Cheeseman KH, Davies MJ, Emery S, Maddix SP, Slater TF: Effects of alpha-tocopherol on carbon tetrachloride metabolism in rat liver microsomes. Free Rad Res Commun 3: 325–330, 1987
Vatassery GT: Oxidation of vitamin E in red cell membranes by fatty acids, hydroperoxides and selected oxidants. Lipids 24: 299–304, 1989
Farris MW, Reed DJ: High performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods in Enzy 143: 101–109, 1989
Gaitonde MK: A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104: 627–633, 1967
Parks DA, Williams TK, Beckman TS: Conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat intestine (a reevaluation). Amer J Physiol 254: G768-G774, 1988
Kraswisz JE, Sharon P, Stenson WF: Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster. Gastroenterol 87: 1344–1350, 1984
Sawchuk KA, Canal D, Slaughter M, Bearman D, O'Connor T, Grosfeld JL: A comparison between fructose 1,6-diphosphate glucose or normal saline infusion and species-specific blood exchange transfusions in the treatment of bowel ischemia. Surgery 100: 665–670, 1986
Batelli MG, DellaCorte E, Stirpe E: XO type D in the intestine and other organs of the rat. Biochem J 126: 747–749, 1972
Granger DN, Rutili G, McCord JM: Superoxide radical in feline intestinal ischemia. Gastroenterol 81: 22–29, 1981
Roy RS, McCord JM:In Oxyradicals and their scavengers systems (Greenwald RA, Cohen G eds). 2, Elsevier, New York, pp 145–153
Yokoyama JS, Beckman TS: Circulating xanthine oxidase-potential mediator of ischemia injury. Amer J Physiol 258: G564-G570, 1990
Nilsson UA, Olsson LI, Thor H, Moldeus P, Fellenius ACB: Detection of oxygen radicals during reperfusion of intestinal cellsin vitro. Free Rad Biol Med 6: 251–259, 1989
Yamamoto M, Shima T, Uozumi R, Sogabe T, Yamada K, Kawasaki T: A possible role of lipid peroxidation in cellular damages caused by cerebral ischemia and the protective effect of vitamin E administration. Stroke 14: 977–982, 1983
Schoenberg MH, Muhe E, Sellin D, Younes M, Schildberg FN, Haglund U: Posthypotensive generation of superoxide free radicals—possible role in the pathogenesis of the intestinal mucosal damage. Acta Chir Scand 150: 301–309, 1984
David H, Siems WG, Ellermann J: Ultrastructure and biochemistry of ischemic damages of small intestinal epithelial cells. Exp Toxic Pathol 44: 325–335, 1992
Rebi S, Lauterburg BH: Hepatic glutathione is regulated by the availability of cysteine and not by feedback inhibition. J Hepatol (Suppl 2) 13: 3–7, 1991
Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN: Role of neutrophils in ischemia/reperfusion-induced microvascular injury. Amer J Physiol 253: H699-H703, 1987
Smith SL, Grisham MB, Manci EA, Granger DN, Kvietys PR: Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroenterol 92: 950–956, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nalini, S., Mathan, M.M. & Balasubramanian, K.A. Oxygen free radical induced damage during intestinal ischemia/reperfusion in normal and xanthine oxidase deficient rats. Mol Cell Biochem 124, 59–66 (1993). https://doi.org/10.1007/BF01096382
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01096382