Abstract
Background
Response to immune checkpoint inhibitors depends on tumor intrinsic properties and also on host factors in the tumour microenvironment including the presence of immune cells (IC). We hypothesized that nivolumab efficacy varies across different metastatic sites.
Methods
We retrospectively analyzed computed tomography scans of patients with metastatic non-small cell lung carcinoma (NSCLC) receiving nivolumab. RECIST 1.1 criteria were applied to assess the overall response rate (ORR) and organ-specific response rate (OSRR).
Results
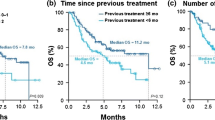
We analyzed 52 patients including 44% females, 58% adenocarcinoma and 8% never smokers. Involved organs had target-lesions in the lung (42%), liver (25%), lymph nodes (56%) and soft tissue (13%) and non-target lesions in the bones (23%). ORR and disease control rate (DCR) were 20% and 45%, respectively. Median overall survival, progression-free survival and duration of response were 11.9, 2.3 and 10.3 months. OSRR and organ-specific DCR (OSDCR) were 28% and 90% in lymph nodes, 8% and 54 in the liver, and 9% and 55% in lung metastases. Nine out of 12 patients with bone metastases had progressive lesions. The cumulative incidence probability of organ-specific progression at 6 months was 14% in lymph nodes, 42% in the liver, 36% in lung metastases and 26% in the primary tumor, 29% in soft tissue and 33% in adrenal metastases.
Conclusion
In conclusion, the efficacy of immunotherapy is dependent on the metastatic location. Treatment appears more active in lymph nodes compared to other organ sites such as liver, adrenals and bone. Future strategies may include additional local treatment in case of oligoprogression in these organs in patients with otherwise sustained treatment benefit.


Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cells
- CR:
-
Complete remission
- CT:
-
Computed
- DCR:
-
Disease control rate
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- OSDCR:
-
Organ-specific disease control rate
- OSRR:
-
Organ-specific response rate
- PD:
-
Progressive disease
- PD-L1:
-
Programmed death ligand 1
- PFS:
-
Progression-free survival
- PR:
-
Partial remission
- SD:
-
Stable disease
- SUV:
-
Standard uptake value
- TME:
-
Tumor microenvironment
References
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Herbst RS, Baas P, Kim DW et al (2015) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 87:1540–1550
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837–1846
Brahmer J et al (2017) Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer: clinical characteristics of long-term survivors (Abstr 003 AACR 2017)
Aguiar PN Jr, Perry LA, Penny-Dimri J et al (2017) The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol 28:2256–2263
Matter-Walstra K, Schwenkglenks M, Aebi S et al (2016) A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol 11:1846–1855
Ribas A (2015) Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov 5:915–919
Ilie M, Long-Mira E, Bence C et al (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27:147–153
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847–856
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348:56–61
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541:321–330
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571
Diem S, Hasan Ali O, Ackermann CJ et al. Tumor infiltrating lymphocytes in lymph node metastases of stage III melanoma correspond to response and survival in nine patients treated with ipilimumab at the time of stage IV disease. Cancer Immunol Immunother 2017
Li F, Tian Z (2013) The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 10:292–302
Tumeh PC, Hellmann MD, Hamid O et al (2017) Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 5:417–424
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Nishino M, Ramaiya NH, Chambers ES et al (2016) Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer 4:84
(2015) The lungs at the frontlines of immunity. Nat Immunol 16(1):17. https://doi.org/10.1038/ni.3069
Bornstein SR, Rutkowski H (2002) The adrenal hormone metabolism in the immune/inflammatory reaction. Endocr Res 28:719–728
Kanczkowski W, Sue M, Zacharowski K et al (2015) The role of adrenal gland microenvironment in the HPA axis function and dysfunction during sepsis. Mol Cell Endocrinol 408:241–248
Weickhardt AJ, Scheier B, Burke JM et al (2012) Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 7:1807–1814
Shaverdian N, Lisberg AE, Bornazyan K et al (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895–903
Theelen W, Peulen H, Lalezari F et al (2018) Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: the PEMBRO-RT study. ASCO 2018, J Clin Oncol 36(suppl):abstr 9023
Huang AC, Postow MA, Orlowski RJ et al (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60–65
Niemeijer A-L, Smit E, Van Dongen G et al (2017) Whole body and PD-L1 PET in patients with NSCLC. Annals of Oncology. https://doi.org/10.1093/annonc/mdx380.008
Acknowledgements
Institutional grant was received by BMS for data collection and statistical analysis.
Author information
Authors and Affiliations
Contributions
Conception and design: SS, SD, MF. Collection and assembly of data: SS, SD, MK. Data analysis and interpretation. Radiology: LD, SL. Statistics: DK, QL. Overall Interpretation: SS, SD, MF. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors. The study was approved by the local Research Ethics Committee (Ethikkommission Ostschweiz-EKOS).
Rights and permissions
About this article
Cite this article
Schmid, S., Diem, S., Li, Q. et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother 67, 1825–1832 (2018). https://doi.org/10.1007/s00262-018-2239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2239-4