Abstract
Background
This study focuses on probiotics in patients with severe acute pancreatitis. It assesses whether enteral feeding with probiotics use reduces infected necrosis and death in severe acute pancreatitis.
Materials and methods
We searched the Cochrane Library, Medline, Embase, and Chinese Biomedicine Database. Quality assessment and data extraction were done by two reviewers independently. The statistical analysis was performed by RevMan4.2.10 software. The result was expressed with odds ratio (OR) for the categorical variable.
Results
Four studies were included. The result showed that using probiotics could not reduce the risk of infection pancreatic necrosis (OR = 0.56, 95% CI [0.13, 2.35]). There is no significant difference between the two groups in mortality (OR = 0.83, 95% CI [0.14, 4.83]), the mean duration of hospital (WMD = −1.20, 95% CI [−13.13, 10.92]) and the required operation (OR = 0.59, 95% CI [0.11, 3.07]).
Conclusion
The present study showed the enteral feeding with probiotic could not reduce the infected necrosis and mortality. Future large-scale, high-quality, placebo-controlled, double-blind trials are needed.
Similar content being viewed by others
Background
Acute pancreatitis is a potentially life-threatening condition that is characterized clinically by abdominal pain, nausea, and vomiting and biochemically by elevations of lipase and/or amylase [1]. The incidence of acute pancreatitis appears to be rising. Incidence ranges in the UK from 150 to 420 cases per million population [2, 3]. In the US, it is increasing by about 5% per year [4]. Severe acute pancreatitis is frequently associated with necrosis of the gland, and the principal late complication is infection of the necrosis [5]. In addition to early mortality from organ failure, late infectious complications have a mortality of about 10–30% [6]. Small bowel bacterial overgrowth and subsequent bacterial translocation are held responsible for the majority of these infections [7]. Attempts to diminish infectious complications with prophylactic antibiotics have been unsuccessful [8, 9]. Another strategy using probiotics has been proposed to reduce the infection of necrosis by intestinal bacteria.
According to Salminen [10], a probiotic is a live microbial culture or cultured dairy product, which beneficially influences the health and nutrition of the host. Now, certain strains of probiotic bacteria might prevent infectious complications by reducing small bowel bacterial overgrowth, restoring gastrointestinal barrier function and modulating the immune system [4, 11, 12]. Some trials with enteral probiotics have shown a significant reduction of infectious complications in acute pancreatitis [13, 14]. But these studies were small, and had some statistical flaws. More recently, a double-blind, placebo-controlled randomized multicenter trial demonstrates that probiotic strains did not reduce the risk of infectious complications and was associated with an increased risk of mortality [4]. It also had some shortcomings.
We did a meta-analysis of randomized controlled trials (RCTs) to assess the effects of probiotic in patients with predicted severe acute pancreatitis.
Method
Search strategy and selection criteria
The Cochrane Review Group’s standard search strategy was used. We searched the Cochrane Library (2008, 1 issue), Medline (1966–2008/3), Embase (1974–2008/3), and China Biomedical Literature Database (1978–2008/3), China Journal Fulltext Database (1994–2008/3), Chinese Scientific Journals Full text Database (1989–2008/3). No language restriction was applied. The search terms would be used including: probiotic, lactobacillus, clostridium butyricum, pediococcus pentosaceus, pancreatitis. We also hand-searched the reference lists of every primary study for additional publications. Further searches were done by reviewing abstract booklets and review articles.
All randomized trials, published and unpublished, which tested the effect of enteral feeding prophylactic probiotic use for patients with severe acute pancreatitis. Studies must be published as full-length articles or letters in peer-reviewed journals. For duplicate publications, the smaller dataset was excluded. Two reviewers (Sun, Tian) independently evaluated studies for eligibility. Studies must have had objective outcome measures, otherwise they were excluded from the review. The outcome measures were the infected pancreatic necrosis, other infectious complications (including chest infection, urinary tract infection, and systemic inflammatory response syndrome), mortality, the mean duration of hospital stay, and required surgical operation.
Data extraction
Data were extracted by two of us (Sun and Yang). The results were compared and disagreements resolved by consensus. From each eligible trial, we recorded the first author, publication year, journal, country of origin, study design, mean age of participants, intervention, outcome, et al.
Quality assessment
We assessed the method of every study using a four-item checklist, namely, reporting of randomization method, allocation concealment, blinding of outcome assessment, and completeness of follow-up. The criteria were drawn from the Cochrane Collaboration guidelines [15]. To assess the effect of trial quality on the effect size, sensitivity analysis was done by comparison of studies that fulfilled quality criteria with those that did not.
Statistical analysis
We analyzed the data using Review Manager (version 4.2.10) and extracted and pooled data for summary estimates. We expressed results for dichotomous outcomes as odds ratio (OR) with 95% confidence intervals (CI) and continuous outcomes as weighted mean difference or standard mean difference. We used the chi square statistic to assess heterogeneity between trials and the I 2 statistic to assess the extent of inconsistency. We used a fixed-effect model for calculations of summary estimates and their 95% CI, unless there was significant heterogeneity, in which case results were confirmed using a random-effects statistical model. Subgroup analyses were intended to explore important clinical differences among trials that might be expected to alter the magnitude of treatment effect.
Role of funding source
No funding source was involved in the conception or development of the study. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Result
The electronic searches yielded 69 items from PubMed, 152 from Embase, seven from the Cochrane Central Register of Controlled Trials, and 30 from Chinese Biomedicine database. Publication dates ranged from 2002 to 2007. After reviewing each publication, we identified seven original studies (Fig. 1). There were four studies [14, 16–18] from the same place—Petz Aladár Megyei Oktató Kórház, Hungary. After reading the article, we found there were two studies [14, 16] really. Two studies [4, 7] were from the same center. One [7] was the design of another [4]. So at last, four studies [4, 14, 16, 19] (428 patients) were included.
Table 1 contains specific information on study design, random methods, samples size, etiology, intervention, and follow up. One study [14] pointed out patients with biliary tract disease were excluded. Only one [4] of the four studies pointed out the randomization was done with a computer-generated permuted-block sequence. Double-blind trials were described in two studies [4, 16]. All of the studies gave us the detailed information about the baselines. Follow-up was used in one study [4], others were not described.
Infected pancreatic necrosis
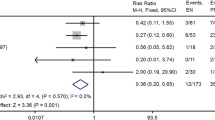
Three [4, 14, 16] of the studies reported the infected pancreatic necrosis. The random effects model was used because of the heterogeneity(I 2 = 63.5%, p = 0.06). Result showed there was no significant difference of infected pancreatic necrosis between enteral feeding with probiotic and without probiotic (OR = 0.56, 95% CI [0.13, 2.35]) (Fig. 2).
Other infectious complications
Other infectious complications showed no significant difference of chest infection (OR = 1.24, 95% CI [0.68, 2.24]), urinary tract infection (OR = 0.70, 95% CI [0.18, 2.75]) and systemic inflammatory response syndrome (OR = 0.64, 95% CI [0.25, 1.61]). However, there was no heterogeneity of the studies (Fig. 3).
Mortality
Figure 4 showed the mortality of the comparing enteral feeding with probiotic vs without probiotic. Three studies [4, 14, 16] were included. The result showed the heterogeneity of mortality across studies (I 2 = 73.2%, p = 0.02), which indicated (OR = 0.83, 95% CI [0.14, 4.83]). To account for heterogeneity in the studied populations, they were pooled.
The mean duration of hospital
Four studies reported the mean duration of hospital stay. But two [14, 16] of these did not tell us the reasonable date. The analysis result of the mean duration of hospital stay was demonstrated in Fig. 5. The heterogeneity also was showed across studies (I 2 = 85.7%, p = 0.008). And for acute pancreatitis, probiotic could not change the mean duration of hospital stay (OR = 1.20, 95% CI [−13.33, 10.92]).
Requiring surgical operation
The analysis result of requiring surgical operation with enteral feeding of using probiotic vs not using probiotic was shown in Fig. 6. This result was not statistically significant (OR = 0.59, 95% CI [0.11, 3.07]), and between-study heterogeneity was present (I 2 = 78.8%, p = 0.009).
Discussion
Severe acute pancreatitis is frequently associated with necrosis of the gland, and the principal late complication is infection of the necrosis. The gut barrier plays an important role in severe acute pancreatitis; in fact, gut barrier integrity prevents bacteria translocation resulting from an atrophic and leaky gut, and reduces the systemic inflammatory syndrome of the pancreatitis from gut atrophy [5]. The maintenance of gut barrier integrity is one of the goals in the treatment of severe acute pancreatitis.
Unfortunately, two meta-analyses [20, 21] and two double-blind, placebo-controlled trials [22, 23] have failed to show a beneficial effect about the antibiotic prophylaxis for the patients with severe acute pancreatitis. So another method using probiotics has been proposed to reduce the infection of necrosis by intestinal bacteria. One experimental study is effective in reducing microbial translocation in pancreatitis [24]. The studies of Oláh et al. 2002 [14] and Oláh et al. 2007 [16] found enteral feeding with probiotic was effective in reducing infections and mortality, as well as reducing the number of surgical interventions. In contrast, Besselink et al. 2008 [4] found probiotic did not reduce the risk of infectious complications and was associated with an increased risk of mortality. But the present results disagreed with all of these studies. It has shown that enteral feeding with probiotic had not produced a satisfactory effectiveness for patients with acute pancreatitis. Using probiotic could not reduce the risk of infections and mortality, nor change the mean duration of hospital stay and decrease the cases of requiring surgical operation.
The results of Besselink et al. 2008 [4] were different from other studies. Why? We might consider whether the composition of the product or the doses used explained the effects noted. The daily dose was similar in these studies, although the combination of probiotic strains administered was different. There are hundreds of different strains of lactic acid bacteria in the human gut, but only a few might act as probiotics [9]. The six probiotic strains used in Besselink et al 2008 [4] were selected from 69 different probiotic bacteria. In the study of Oláh et al. 2007 [16], only four probiotic strains were used, and in Oláh et al. 2002 [14], only Lactobacillus plantarum 299. The different probiotic strains might make the different effect. For mortality, the following reason might be considered: A meta-analysis showing that although immunonutrition in elective surgical patients reduced the infection rate, it increased mortality in critically ill patients [25]. This effect was seen only in studies of high methodological quality and the reasons for the increased mortality could not be identified [4]. Mortality increased when glutamine was administered after the induction of a low flow state [26], although glutamine could protect against the effects of bowel ischemia [27]. Apparently, in the study of Besselink et al., there was reason for concern about administration of potent immunonutritional supplements in the presence of a low flow state, or more generally, in the critically ill [4].
Some criticized Oláh et al. 2002 [14] because of the exclusion of biliary pancreatitis patients, small number of participants, and some statistical flaws [7]. In Oláh et al. 2007, done by the same research group in 62 patients with predicted severe pancreatitis, the number of the participants was relatively small, although biliary pancreatitis patients were included. The Chinese study Li 2007 [19] did not tell us the details of the inclusion of the patients and some outcome measures were not suitable, except the small number of participants. Fortunately, Besselink et al. 2008 [4] involving 296 patients was well planned with sound randomization. However, one result raises questions about the success of randomisation. Rates of organ failure were similar in both groups before randomization (probiotic group 5.9% and placebo group 3.5%). Similarly, there were no differences in organ failure after randomization (13.8% vs 11.1%). But was there any difference on the particular day of randomization? Organ failure during any onset was more common in patients taking probiotics than in those taking placebo (27.0% vs 16.0%, p = 0.02). We calculated that if organ failures after randomization are omitted, more patients in the probiotic group had organ failures before or during the day of the first dose than did those in the placebo group (13.2% vs 4.9%, p = 0.01). Thus, the two groups might have differed from each other before the probiotics could have had an effect [9].
This study also have its limitation. The number of the included studies is relatively small, except Besselink et al. 2008 [4] The small number of participants, as well as the low quality of three studies, might not allow for a reliable conclusion. Only one [4] of the four included studies pointed out randomization was done with a computer-generated permuted-block sequence. Two [4, 16] mentioned double blinding, so these would produce high-performance bias and measuring bias. Future research should clearly spell out how to implement randomization and blinding.
The heterogeneity of some variables in this study is worthy of comment. Four of five variables exhibited significant heterogeneity (I 2 more than 60%). Explanations may include the following. First, the different probiotics were performed by different physicians in each hospital. Second, the trials had different inclusion–exclusion criteria and sample sizes. Finally, there were differences in the study design and operative techniques.
In order to overcome the methodological limitations of the previous studies that we reviewed in this meta-analysis, we make the following suggestions for the design and reporting of future RCTs: participant enrollment should meet a common inclusion–exclusion criteria with severe pancreatic necrosis and the sample size must be sufficient to detect a significant difference. More details of the trial, such as randomization and blinding, should be described.
Conclusion
The present study showed that enteral feeding with probiotic could not reduce the infected necrosis and mortality. Two groups were similar in duration of hospital stay or the need for surgical operation. Future large-scale, high-quality, placebo-controlled, double-blind trials are still required to clarify the issues of the effect of probiotic in severe acute pancreatitis.
References
Reisler RB, Murphy RL, Redfield RR (2005) Incidence of pancreatitis in HIV-1-infected individuals enrolled in 20 adult AIDS clinical trials group studies: lessons learned. J Acquir Immune Defic Syndr 39:159–166
McKay CJ, Evans S, Sinclair M et al (1999) High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg 86:1302–1305 doi:10.1046/j.1365-2168.1999.01246.x
Toh SK, Phillips S, Johnson CD (2000) A prospective audit against national standards of the presentation and management of acute pancreatitis in the South of England. Gut 46:239–243 doi:10.1136/gut.46.2.239
Besselink MGH, van Santvoort HC, Buskens E et al (2008) Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Dutch Acute Pancreatitis Study Group Lancet 371:651–659 doi:10.1016/S0140-6736(08)60207-X
Pezzilli R, Fantini L (2006) Probiotics and severe acute pancreatitis. JOP 7:92–93
UK Working Party on Acute Pancreatitis (2005) UK guidelines for the management of acute pancreatitis. Gut 54(suppl 3):1–9
Besselink MG, Timmerman HM, Buskens E, Nieuwenhuijs VB, Akkermans LM, Gooszen HG, Dutch Acute Pancreatitis Study Group (2004) Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial. BMC Surg 4:12 ISRCTN38327949 doi:10.1186/1471-2482-4-12
Mazaki T, Ishii Y, Takayama T (2006) Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg 93:674–684 doi:10.1002/bjs.5389
Sand J, Nordback I (2008) Probiotics in severe acute pancreatitis. Lancet 371:634–635 doi:10.1016/S0140-6736(08)60284-6
Salminen S (1996) Uniqueness of probiotic strains. IDF Nutrition Newsletter 5:16–18
Bengmark S (1998) Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 42:2–7
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361:512–519 doi:10.1016/S0140-6736(03)12489-0
Kecskés G, Belágyi T, Oláh A (2003) Early jejunal nutrition with combined pre- and probiotics in acute pancreatitis—prospective, randomized, double-blind investigations. Magy Seb 56:3–8
Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S (2002) Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg 89:1103–1107 doi:10.1046/j.1365-2168.2002.02189.x
Collaboration TC (2006) Cochrane handbook for systematic reviews of interventions 4.2.6: The Cochrane Collaboration.
Oláh A, Belágyi T, Pótó L, Romics L Jr, Bengmark S (2007) Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology 54:590–594
Oláh A, Belágyi T, Issekutz A, Olgyai G (2005) Combination of early nasojejunal feeding with modern synbiotic therapy in the treatment of severe acute pancreatitis (prospective, randomized, double-blind study). Magy Seb 58:173–178
Kecskés G, Belágyi T, Oláh A (2003) Early jejunal nutrition with combined pre- and probiotics in acute pancreatitis—prospective, randomized, double-blind investigations. Magy Seb 56:3–8
Li YM (2007) Adjuvant therapy for probiotics in patients with severe acute pancreatitis: An analysis of 14 cases. World Chinese Journal of Digestology 15:302–304
De Vries AC, Besselink MG, Buskens E (2007) Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology 7:531–538 doi:10.1159/000108971
Mazaki T, Ishii Y, Takayama T (2006) Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg 93:674–684 doi:10.1002/bjs.5389
Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T et al (2007) Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg 245:674–683 doi:10.1097/01.sla.0000250414.09255.84
Isenmann R, Runzi M, Kron M, Kahl S, Kraus D, Jung N, German Antibiotics in Severe Acute Pancreatitis Study Group (2004) Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology 126:997–1004 doi:10.1053/j.gastro.2003.12.050
Mangiante G, Colucci G, Canepari P, Bassi C, Nicoli N, Casaril A et al (2001) Lactobacillus plantarum reduces infection of pancreatic necrosis in experimental acute pancreatitis. Dig Surg 18:47–50 doi:10.1159/000050096
Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U (2001) Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286:944–953 doi:10.1001/jama.286.8.944
Omata J, Fukatsu K, Ueno C, Maeshima Y, Saitoh D, Mochizuki H (2007) Intraluminal glutamine administration during ischemia worsens survival after gut ischemia-reperfusion. J Surg Res 132:260–264 doi:10.1016/j.jss.2006.12.004
Sukhotnik I, Khateeb K, Mogilner JG, Helou H, Lurie M, Coran AG et al (2007) Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following ischemia-reperfusion injury in the rat. Dig Dis Sci 52:1497–1504 doi:10.1007/s10620-006-9629-8
Acknowledgment
Thanks a lot to the co-authors and all authors, who have contributed significantly. All authors are in agreement with the content of the manuscript. The copyright will be sent to your Journal.
I appreciate the China Evidence-based Medicine Center.
Conflict of interest:
There is no financial support or relationships that may pose conflict of interest in this study paper.
Funding sources:
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, S., Yang, K., He, X. et al. Probiotics in patients with severe acute pancreatitis: a meta-analysis. Langenbecks Arch Surg 394, 171–177 (2009). https://doi.org/10.1007/s00423-008-0379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0379-2