-
PDF
- Split View
-
Views
-
Cite
Cite
Robert E. Mansel, Lesley Fallowfield, Mark Kissin, Amit Goyal, Robert G. Newcombe, J. Michael Dixon, Constantinos Yiangou, Kieran Horgan, Nigel Bundred, Ian Monypenny, David England, Mark Sibbering, Tholkifl I. Abdullah, Lester Barr, Utheshtra Chetty, Dudley H. Sinnett, Anne Fleissig, Dayalan Clarke, Peter J. Ell, Randomized Multicenter Trial of Sentinel Node Biopsy Versus Standard Axillary Treatment in Operable Breast Cancer: The ALMANAC Trial, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 9, 3 May 2006, Pages 599–609, https://doi.org/10.1093/jnci/djj158
Close - Share Icon Share
Abstract
Background: Sentinel lymph node biopsy in women with operable breast cancer is routinely used in some countries for staging the axilla despite limited data from randomized trials on morbidity and mortality outcomes. We conducted a multicenter randomized trial to compare quality-of-life outcomes between patients with clinically node-negative invasive breast cancer who received sentinel lymph node biopsy and patients who received standard axillary treatment. Methods: The primary outcome measures were arm and shoulder morbidity and quality of life. From November 1999 to October 2003, 1031 patients were randomly assigned to undergo sentinel lymph node biopsy (n = 515) or standard axillary surgery (n = 516). Patients with sentinel lymph node metastases proceeded to delayed axillary clearance or received axillary radiotherapy (depending on the protocol at the treating institution). Intention-to-treat analyses of data at 1, 3, 6, and 12 months after surgery are presented. All statistical tests were two-sided. Results: The relative risks of any lymphedema and sensory loss for the sentinel lymph node biopsy group compared with the standard axillary treatment group at 12 months were 0.37 (95% confidence interval [CI] = 0.23 to 0.60; absolute rates: 5% versus 13%) and 0.37 (95% CI = 0.27 to 0.50; absolute rates: 11% versus 31%), respectively. Drain usage, length of hospital stay, and time to resumption of normal day-to-day activities after surgery were statistically significantly lower in the sentinel lymph node biopsy group (all P <.001), and axillary operative time was reduced ( P = .055). Overall patient-recorded quality of life and arm functioning scores were statistically significantly better in the sentinel lymph node biopsy group throughout (all P ≤.003). These benefits were seen with no increase in anxiety levels in the sentinel lymph node biopsy group ( P >.05). Conclusion: Sentinel lymph node biopsy is associated with reduced arm morbidity and better quality of life than standard axillary treatment and should be the treatment of choice for patients who have early-stage breast cancer with clinically negative nodes.
The online version of this article has been published under an Open Access model. Users are entitled to use, reproduce, disseminate, or display the Open Access version of this article for non-commercial purposes provided that: the original authorship is properly and fully attributed; the Journal and Oxford University Press are attributed as the original place of publication with the correct citation details given; if an article is subsequently reproduced or disseminated not in its entirety but only in part or as a derivative work this must be clearly indicated. For commercial re-use, please contact: journals.permissions@oxfordjournals.org .
The increasing use of breast-conserving therapy has reduced the major morbidity of mastectomy, thereby focusing greater attention on the sequelae of axillary surgery. For patients with invasive breast cancer, the standard approach to axillary surgery has been axillary lymph node dissection. This approach consumes considerable resources and causes both acute and late morbidities for the patient, with complications that include lymphedema, pain, numbness, and limited shoulder movement ( 1 – 5 ) . Moreover, most women with early-stage breast cancer are node negative, and axillary dissection in these women exposes them to the complications of this procedure, with no benefit.
Sentinel lymph node biopsy is a minimally invasive alternative to axillary lymph node dissection as a way to stage breast cancer in clinically node-negative patients. A sentinel lymph node is any lymph node that receives direct lymphatic drainage from a primary tumor site. Nonrandomized studies of sentinel node biopsy followed by axillary lymph node dissection have demonstrated that one or more sentinel lymph nodes can be identified in more than 90% of patients with invasive breast cancer, with a false-negative rate of 10% or less ( 7 , 8 ) . Sentinel lymph node biopsy appears to reliably identify node-negative patients who can be spared the morbidity resulting from axillary lymph node dissection ( 9 ) . A few small, predominantly observational studies have reported on quality-of-life benefits associated with sentinel lymph node biopsy, but these benefits have not been confirmed in randomized trials using well-validated survey instruments ( 10 – 17 ) . Also, none of these studies considered the potential increased anxiety experienced by patients undergoing sentinel lymph node biopsy because of the possibility that some involved nodes might be missed or because of the need for further surgery if a sentinel lymph node is found to be involved.
Results from multicenter randomized trials that focus on postoperative morbidity and relapse-free and overall survival following sentinel lymph node biopsy are required before this procedure can be accepted as the standard of care. To date, the only published randomized trial of sentinel lymph node biopsy ( 17 ) was a small, single-institution study that used isotope localization of sentinel lymph nodes. That study did not use a validated quality-of-life assessment and evaluated morbidity only in a subset of 200 patients.
Here we report results from the Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial, in which patients diagnosed with invasive breast cancer were randomly assigned to receive sentinel lymph node biopsy or standard axillary treatment. The primary endpoints were arm morbidity and quality of life, as assessed by multiple measures. The secondary endpoint was the axillary recurrence rate. Local recurrence and survival will be addressed in a later study that includes follow-up data from this trial as well as data from the ongoing National Surgical Adjuvant Bowel and Breast Project (NSABP-32) and the American College of Surgeons Oncology Group (ACOSOG) trials. More results from the quality-of-life study using data from the 829 patients who completed the quality-of-life questionnaires were reported separately ( 18 ) .
P ATIENTS AND M ETHODS
Study Design
The ALMANAC trial was a multicenter randomized study that compared sentinel lymph node biopsy with standard axillary treatment in the management of patients with early-stage clinically node-negative breast cancer ( 7 , 19 ) . The trial is registered with the National Cancer Research Network ( http://www.ncrn.org.uk ) as NCRN Trial ID 843. The randomized phase of this trial was preceded by a validation phase, during which each participating surgeon performed at least 40 sentinel lymph node biopsies that were followed by either axillary lymph node dissection or four-node axillary sampling to stage the axilla, whichever was the current standard staging procedure in that center. Surgeons who identified a sentinel lymph node in at least 90% of their patients with invasive breast cancer and a maximum of two false-negative results for 40 consecutive cases were eligible to participate in the randomized phase of the trial. It took 3–9 months for the eligible surgeons to complete the validation phase, depending on their caseloads ( 7 ) . We have previously reported that increased body mass index of the patient, tumor location other than upper outer quadrant of the breast, and lack of activity on preoperative lymphoscintigraphy are all statistically significantly associated with surgeon failure to find a sentinel lymph node ( 20 ) .
In the randomized phase of the trial, subsequent patients of the eligible surgeons were randomly assigned centrally (Cardiff, UK) by fax, in a 1 : 1 ratio using a computer-generated sequence, to undergo sentinel lymph node biopsy or standard axillary treatment, i.e., level I–III axillary lymph node dissection or four-node axillary sampling ( 21 ) . Patients with metastatic disease in a sentinel lymph node were offered a choice of either delayed axillary lymph node dissection or axillary radiotherapy after discussion with the patient and the multidisciplinary team. This study was overseen by a scientific steering committee and an independent data monitoring committee. Patients were reviewed at 1, 3, 6, 12, and 18 months after surgery. Analysis of follow-up data to 12 months is presented in this paper.
Patients
Patients of either sex who were younger than 80 years and scheduled to have a wide local excision or mastectomy for clinically node-negative invasive breast cancer regardless of tumor size were eligible to participate in the randomized phase of the trial. Written informed consent was required from every participant according to a protocol approved by local ethics committees and in accordance with the Declaration of Helsinki. Exclusion criteria were multicentric cancer; previous ipsilateral breast or axillary surgery other than benign excision biopsy; previous irradiation of the ipsilateral axilla or breast; preexisting limb disease causing swelling; known allergy to human albumin or Patent Blue V; pregnancy or breast feeding; and inability to complete quality-of-life questionnaires in English.
Sentinel Lymph Node Identification and Biopsy
The sentinel lymph node biopsy was performed before the breast tumor was removed according to a standardized protocol that used a radiopharmaceutical compound and a blue dye with routine preoperative lymphoscintigraphy. The times of initiation and completion of the primary tumor excision and of the axillary procedure were prospectively recorded. In brief, 2 mL of the radiopharmaceutical compound technetium (99mTc)–albumin colloid (Nanocoll; GE Healthcare, Little Chalfont, England) was injected at four sites peritumorally on the day before surgery (dose = 40 MBq) or on the day of surgery (dose = 20 MBq), and the injected area was massaged gently for approximately 5 minutes to facilitate lymphatic drainage. Static scintigraphic images, in anterior and oblique projections, were obtained approximately 3 hours after injection of the radiocolloid tracer by using a dual-head gamma camera with a low-energy, high-resolution collimator (4-minute acquisition in a 256 × 256 matrix). The locations of axillary and nonaxillary sentinel lymph nodes were marked on the patient's skin. The patient was given general anesthesia in the operating room, and 3–5 minutes before the first incision was made, 2 mL of Patent Blue V dye (Laboratoire Guerbet, Aulnay-sous-Bois, France) diluted to a total volume of 5 mL with saline was injected peritumorally. Intraoperative identification of sentinel lymph nodes was based on blue dye mapping and handheld gamma probe detection. All blue-stained nodes and nodes with radioactive counts more than 10 times the background count (as measured at the antecubital fossa using the handheld gamma probe) were defined as sentinel lymph nodes. If internal mammary drainage was seen on lymphoscintigraphy, it was recommended (but not required) that these internal mammary sentinel nodes be removed. When no sentinel lymph node could be identified, standard axillary treatment was performed.
After the sentinel lymph node biopsy was completed, either a breast-conserving procedure (92% of patients in each study arm) or a mastectomy (8% of patients in each study arm) was performed to remove the primary tumor (the choice of procedure was decided pre-operatively based on clinical grounds), and tumor specimens were sent for routine pathology.
Pathology Examination
All lymph nodes were examined by standard hematoxylin–eosin staining. Lymph nodes smaller than 5 mm were bisected and stained; those 5 mm or larger were sectioned at 3-mm intervals, and single sections were stained with hematoxylin–eosin. Intraoperative histologic examination or immunohistochemical staining techniques were not used to examine the lymph nodes.
Adjuvant Treatment
Patients were treated with adjuvant radiation therapy and systemic therapy according to standard institutional protocols.
Outcome Assessments
The change in the ipsilateral arm volume at each follow-up visit was expressed as a percent increase from the pretreatment value. Ratios of presurgery to postsurgery arm volumes were compared on a log-transformed scale. Shoulder function (i.e., flexion, abduction, internal rotation, and external rotation) on both sides was assessed by goniometric measurement of arm movement. Changes in function at each follow-up visit were calculated by subtraction. The contralateral arm was used as a control for evaluations of arm volume and shoulder function. Sensory deficits in the ipsilateral upper arm and axilla was assessed by using the pin-prick method. At each visit, the clinician assessed whether sensory loss within the area of the intercostobrachial nerve was absent, mild, or severe.
The infection rate was determined by review of the patient's case notes and by observation of the surgical wounds but was not confirmed in every case by bacteriology.
Quality of life. Quality of life was assessed with the Functional Assessment of Cancer Therapy-Breast + 4 questionnaire (FACT-B+4). The FACT-B is a comprehensive measure of overall quality of life ( 23 ) that contains one item on arm morbidity. Four additional validated arm morbidity items were added to create an enhanced instrument, FACT-B+4, ( 24 ) . FACT-B+4 comprises five subscales: physical well-being (seven items), social well-being (seven items), emotional well-being (six items), functional well-being (seven items), and concerns specific to patients with breast cancer (13 items). The arm functioning subscale ( Appendix I ) comprises the five specific items that are concerned with arm morbidity. Although these five items are included in the functional well-being and breast cancer concerns subscales, they can also be analyzed separately.
FACT-B+4 asks patients to indicate, using a five-point scale from 0 (not at all), 1 (a little bit), 2 (somewhat), 3 (quite a bit), to 4 (very much), to what degree each statement applied over the previous 7 days. Negatively framed statements were reversed after scoring, so that higher scores indicate a better quality of life and lower scores indicate a worse quality of life. Baseline questionnaires administered by research fellows and research nurses at the study centers were completed without assistance by patients before random assignment. Follow-up questionnaires were mailed to patients at 1, 3, 6, 12, and 18 months after surgery by the quality-of-life coordinating center (Cancer Research UK Psychosocial Oncology Group, Brighton, UK).
The primary quality-of-life endpoints were the trial outcome index (TOI) score of the FACT-B+4 questionnaire, and the total arm functioning score. The TOI score is derived from the sum of the scores on the physical and well-being subscales and on the breast cancer concerns subscale. The 27 items have a maximum score of 108, which reflects a good quality of life. A change in TOI score of at least five points is considered to be clinically relevant or a minimally important difference ( 25 , 26 ) . The FACT B trial outcome index is well known and has recently been used in the evaluation of an international breast cancer treatment trial ( 27 ) . The arm functioning subscale items were scored using the same five-point scale used for FACT-B+4 and had a maximum score of 20. Secondary endpoints were the total score for FACT-B+4 (maximum score = 160) and the scores for two individual arm morbidity items related to swelling and numbness.
We used the Spielberger State/Trait Anxiety Inventory (STAI) ( 28 ) to assess state anxiety and trait anxiety of patients at baseline (i.e., before randomization); thereafter, only state anxiety was assessed at the same time points that FACT-B+4 was completed. Level of anxiety is directly proportional to the STAI scores.
Statistical Analysis
The trial was designed to recruit 1260 patients, with the anticipation of obtaining analyzable responses from 1200 patients. For an expected 600 patients in axillary sampling centers, we assumed a conservative successful localization rate of 90% for sentinel lymph node biopsy and node positivity for 30% of these. We assumed a risk of arm swelling of 20% on sampling and 5% following sentinel lymph node biopsy. The power to detect the difference between the resulting event rates of 10.55% and 20% at a 5% level is then 90%, with higher power for the patients in centers that practice axillary clearance.
All analyses reported were performed on an intention-to-treat basis. Analyses of variables such as arm measurements and shoulder angles, which were recorded at baseline and at follow-up visits, were performed by calculating the absolute change at each time point for the ipsilateral arm minus the absolute change for the contralateral arm at the same time point, and those values were compared between the sentinel lymph node biopsy and control groups. The change in the ipsilateral arm volume at each follow-up visit was expressed as a percent increase from the corresponding pretreatment value. Also, the log-transformed change for the ipsilateral arm minus the log-transformed change for the contralateral arm was calculated for each patient at each time point and those values were compared between the sentinel lymph node biopsy and standard axillary treatment groups. Statistical tests used were unpaired t , Mann–Whitney, and chi-square tests according to distributional form. We estimated 95% confidence intervals (CIs) for proportions ( 29 ) , for differences between proportions ( 30 ) , and for relative risks (RRs) ( 31 ) . All statistical tests were two-sided, and P <.05 was considered statistically significant.
R ESULTS
Trial Profile
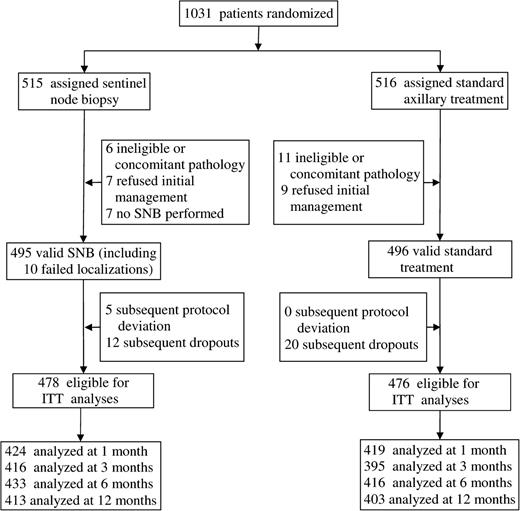
Recruitment to the randomized phase of this study commenced in November 1999. During 2003, it became apparent to some investigators that patients allocated to the sentinel lymph node biopsy arm were experiencing less arm and shoulder morbidity than patients allocated to the axillary lymph node dissection arm. These investigators alerted the principal investigator (R.E.M.) to the loss of equipoise in the trial and the consequent difficulty in obtaining consent for randomization at some centers because of ethical issues. After discussion of this problem by the data monitoring committee, the committee chair reported that there were probably sufficient data accrued to answer the questions posed in the trial. Because the initial power calculations were conservative and it was apparent that large differences were being seen between the study arms, the Steering Committee concluded that the trial should be terminated early. Recruitment ceased on October 31, 2003, by which date 1031 patients from 13 surgeons at 11 centers in the United Kingdom had been randomly assigned to a study arm ( Fig. 1 ). Patients recruited up to July 28, 2003, participated in the quality-of-life assessments. Of the 1031 patients, 17 proved ineligible or had other nonbreast tumors, and 16 refused initial management. For seven patients allocated to the sentinel lymph node biopsy group, this procedure was not performed; thus, 991 patients had either sentinel lymph node biopsy or standard axillary management ( Table 1 ). The two groups of patients were similar with respect to patient and tumor characteristics ( Table 1 ).
Trial flow diagram. SNB = sentinel lymph node biopsy; ITT = intention to treat.
Characteristics of randomly assigned patients in the ALMANAC trial *
| Characteristic . | Sentinel lymph node biopsy (n = 495) . | Standard axillary treatment (n = 496) . |
|---|---|---|
| Mean age, y (SD) | 57.4 (9.9) | 57.9 (9.8) |
| Mean BMI, kg/m 2 (SD) | 26.7 (5.1) | 26.9 (5.7) |
| Sex, No. (%) | ||
| Female | 491 (99.2) | 495 (99.8) |
| Male | 4 (0.8) | 1 (0.2) |
| Screen-detected breast cancer, No. (%) | ||
| Yes | 242 (50) | 222 (46) |
| No | 245 (50) | 261 (54) |
| Axillary nodal metastases found, No. (%) | ||
| Yes | 127 (26) | 116 (23) |
| No | 366 (74) | 380 (77) |
| Type of surgery, No. (%) | ||
| Wide local excision | 457 (92) | 448 (90) |
| Mastectomy | 38 (8) | 48 (10) |
| Tumor size, mm, No. (%) | ||
| ≤20 | 354 (72) | 378 (78) |
| 20.1–50 | 125 (26) | 99 (20) |
| >50 | 10 (2) | 9 (2) |
| Tumor grade, No. (%) | ||
| I | 125 (26) | 131 (26) |
| II | 254 (52) | 237 (48) |
| III | 109 (22) | 121 (25) |
| Tumor pathology, No. (%) | ||
| Invasive ductal | 360 (73) | 356 (72) |
| Invasive lobular | 40 (8) | 43 (9) |
| Other | 95 (19) | 97 (20) |
| Characteristic . | Sentinel lymph node biopsy (n = 495) . | Standard axillary treatment (n = 496) . |
|---|---|---|
| Mean age, y (SD) | 57.4 (9.9) | 57.9 (9.8) |
| Mean BMI, kg/m 2 (SD) | 26.7 (5.1) | 26.9 (5.7) |
| Sex, No. (%) | ||
| Female | 491 (99.2) | 495 (99.8) |
| Male | 4 (0.8) | 1 (0.2) |
| Screen-detected breast cancer, No. (%) | ||
| Yes | 242 (50) | 222 (46) |
| No | 245 (50) | 261 (54) |
| Axillary nodal metastases found, No. (%) | ||
| Yes | 127 (26) | 116 (23) |
| No | 366 (74) | 380 (77) |
| Type of surgery, No. (%) | ||
| Wide local excision | 457 (92) | 448 (90) |
| Mastectomy | 38 (8) | 48 (10) |
| Tumor size, mm, No. (%) | ||
| ≤20 | 354 (72) | 378 (78) |
| 20.1–50 | 125 (26) | 99 (20) |
| >50 | 10 (2) | 9 (2) |
| Tumor grade, No. (%) | ||
| I | 125 (26) | 131 (26) |
| II | 254 (52) | 237 (48) |
| III | 109 (22) | 121 (25) |
| Tumor pathology, No. (%) | ||
| Invasive ductal | 360 (73) | 356 (72) |
| Invasive lobular | 40 (8) | 43 (9) |
| Other | 95 (19) | 97 (20) |
Numbers of patients in some categories total to less than 495 (sentinel lymph node biopsy) or 496 (standard treatment) because of missing data. SD = standard deviation; BMI = body mass index.
Characteristics of randomly assigned patients in the ALMANAC trial *
| Characteristic . | Sentinel lymph node biopsy (n = 495) . | Standard axillary treatment (n = 496) . |
|---|---|---|
| Mean age, y (SD) | 57.4 (9.9) | 57.9 (9.8) |
| Mean BMI, kg/m 2 (SD) | 26.7 (5.1) | 26.9 (5.7) |
| Sex, No. (%) | ||
| Female | 491 (99.2) | 495 (99.8) |
| Male | 4 (0.8) | 1 (0.2) |
| Screen-detected breast cancer, No. (%) | ||
| Yes | 242 (50) | 222 (46) |
| No | 245 (50) | 261 (54) |
| Axillary nodal metastases found, No. (%) | ||
| Yes | 127 (26) | 116 (23) |
| No | 366 (74) | 380 (77) |
| Type of surgery, No. (%) | ||
| Wide local excision | 457 (92) | 448 (90) |
| Mastectomy | 38 (8) | 48 (10) |
| Tumor size, mm, No. (%) | ||
| ≤20 | 354 (72) | 378 (78) |
| 20.1–50 | 125 (26) | 99 (20) |
| >50 | 10 (2) | 9 (2) |
| Tumor grade, No. (%) | ||
| I | 125 (26) | 131 (26) |
| II | 254 (52) | 237 (48) |
| III | 109 (22) | 121 (25) |
| Tumor pathology, No. (%) | ||
| Invasive ductal | 360 (73) | 356 (72) |
| Invasive lobular | 40 (8) | 43 (9) |
| Other | 95 (19) | 97 (20) |
| Characteristic . | Sentinel lymph node biopsy (n = 495) . | Standard axillary treatment (n = 496) . |
|---|---|---|
| Mean age, y (SD) | 57.4 (9.9) | 57.9 (9.8) |
| Mean BMI, kg/m 2 (SD) | 26.7 (5.1) | 26.9 (5.7) |
| Sex, No. (%) | ||
| Female | 491 (99.2) | 495 (99.8) |
| Male | 4 (0.8) | 1 (0.2) |
| Screen-detected breast cancer, No. (%) | ||
| Yes | 242 (50) | 222 (46) |
| No | 245 (50) | 261 (54) |
| Axillary nodal metastases found, No. (%) | ||
| Yes | 127 (26) | 116 (23) |
| No | 366 (74) | 380 (77) |
| Type of surgery, No. (%) | ||
| Wide local excision | 457 (92) | 448 (90) |
| Mastectomy | 38 (8) | 48 (10) |
| Tumor size, mm, No. (%) | ||
| ≤20 | 354 (72) | 378 (78) |
| 20.1–50 | 125 (26) | 99 (20) |
| >50 | 10 (2) | 9 (2) |
| Tumor grade, No. (%) | ||
| I | 125 (26) | 131 (26) |
| II | 254 (52) | 237 (48) |
| III | 109 (22) | 121 (25) |
| Tumor pathology, No. (%) | ||
| Invasive ductal | 360 (73) | 356 (72) |
| Invasive lobular | 40 (8) | 43 (9) |
| Other | 95 (19) | 97 (20) |
Numbers of patients in some categories total to less than 495 (sentinel lymph node biopsy) or 496 (standard treatment) because of missing data. SD = standard deviation; BMI = body mass index.
A total of 37 patients (4%) were excluded because of substantial protocol deviation or because they dropped out of the study (i.e., there were no data available for analysis), leaving 954 patients available for intention-to-treat analyses of efficacy outcomes ( Tables 2 and 3 ).
Postoperative arm morbidity after sentinel node biopsy and standard axillary treatment in the ALMANAC trial *
| Arm morbidity assessment . | Sentinel lymph node biopsy (n = 478 ) Time after surgery (mo) . | . | . | . | Standard axillary treatment (n = 476) Time after surgery (mo) . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 3 . | 6 . | 12 . | 1 . | 3 . | 6 . | 12 . | P . | ||||||
| Lymphedema | |||||||||||||||
| Self-assessment, No. (%) | |||||||||||||||
| None | 414 (97) | 397 (95) | 413 (96) | 392 (95) | 369 (88) | 334 (85) | 343 (83) | 350 (87) | |||||||
| Mild | 13 (3) | 16 (4) | 17 (4) | 16 (4) | 43 (10) | 49 (12) | 58 (14) | 43 (11) | |||||||
| Moderate or severe | 1 ( 0.2) | 4 (1) | 2 (0.5) | 4 (1) | 7 (2) | 12 (3) | 13 (3) | 10 (2) | <.001 † | ||||||
| Mean change in arm volume (95% CI) ‡ | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | <.005 § | ||||||
| Sensory deficit | |||||||||||||||
| Self-assessment, No. (%) | 74/411 (18) | 81/407 (20) | 69/423 (16) | 46/407 (11) | 252/409 (62) | 209/388 (54) | 178/411 (43) | 124/401 (31) | <.001 ‖ | ||||||
| Median area of sensory loss, cm 2 (range) ¶ | 32 (2–254) | 48 (0–327) | 32 (0–201) | 59 (0.2–342) | 40 (1–489) | 47 (0–1139) | 39 (0.4–2827) | 35 (0.8–1013) | >.1 # | ||||||
| Clinician assessment of ICB nerve damage, No. (%) | |||||||||||||||
| None | 351 (86) | 343 (86) | 350 (85) | 365 (91) | 231 (59) | 233 (62) | 251 (64) | 264 (69) | <.001 ** | ||||||
| Mild | 52 (13) | 50 (13) | 56 (14) | 30 (8) | 151 (39) | 130 (35) | 133 (34) | 115 (30) | |||||||
| Severe | 6 (1) | 4 (1) | 4 (1) | 5 (1) | 10 (3) | 10 (3) | 10 (3) | 5 (1) | |||||||
| Mean change in shoulder function, degrees (95% CI) †† | |||||||||||||||
| Flexion | 5.8 (4.3 to 7.4) | 2.0 (0.9 to 3.2) | 2.0 (0.7 to 3.3) | 2.7 (1.2 to 4.2) | 9.8 (7.9 to 11.7) | 3.7 (2.0 to 5.4) | 1.6 (0.2 to 3.0) | 0.1 (−1.2 to 1.5) | .004 ‡‡ | ||||||
| Abduction | 6.5 (4.5 to 8.6) | 1.9 (0.5 to 3.4) | 1.5 (−0.1 to 3.1) | 2.5 (0.6 to 4.4) | 12.9 (10.7 to 15.2) | 4.2 (2.4 to 6.1) | 2.3 (0.7 to 4.0) | 1.9 (0.2 to 3.5) | .001 ‡‡ | ||||||
| External rotation | 0.7 (−0.4 to 1.8) | 0.2 (−0.9 to 1.3) | 0.6 (−0.7 to 1.9) | 0.6 (−0.8 to 2.0) | 1.2 (0.2 to 2.3) | 1.2 (0.0 to 2.3) | 1.0 (−0.2 to 2.1) | 0.7 (−0.8 to 2.1) | .18 ‡‡ | ||||||
| Internal rotation | 0.4 (−0.9 to 1.6) | 1.0 (−0.2 to 2.3) | 0.2 (−1.2 to 1.5) | 1.7 (0.4 to 3.0) | 0.9 (−0.4 to 2.1) | 0.7 (−0.5 to 2.0) | 0.8 (−0.6 to 2.1) | 0.4 (−1.0 to 1.8) | .31 ‡‡ | ||||||
| Arm morbidity assessment . | Sentinel lymph node biopsy (n = 478 ) Time after surgery (mo) . | . | . | . | Standard axillary treatment (n = 476) Time after surgery (mo) . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 3 . | 6 . | 12 . | 1 . | 3 . | 6 . | 12 . | P . | ||||||
| Lymphedema | |||||||||||||||
| Self-assessment, No. (%) | |||||||||||||||
| None | 414 (97) | 397 (95) | 413 (96) | 392 (95) | 369 (88) | 334 (85) | 343 (83) | 350 (87) | |||||||
| Mild | 13 (3) | 16 (4) | 17 (4) | 16 (4) | 43 (10) | 49 (12) | 58 (14) | 43 (11) | |||||||
| Moderate or severe | 1 ( 0.2) | 4 (1) | 2 (0.5) | 4 (1) | 7 (2) | 12 (3) | 13 (3) | 10 (2) | <.001 † | ||||||
| Mean change in arm volume (95% CI) ‡ | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | <.005 § | ||||||
| Sensory deficit | |||||||||||||||
| Self-assessment, No. (%) | 74/411 (18) | 81/407 (20) | 69/423 (16) | 46/407 (11) | 252/409 (62) | 209/388 (54) | 178/411 (43) | 124/401 (31) | <.001 ‖ | ||||||
| Median area of sensory loss, cm 2 (range) ¶ | 32 (2–254) | 48 (0–327) | 32 (0–201) | 59 (0.2–342) | 40 (1–489) | 47 (0–1139) | 39 (0.4–2827) | 35 (0.8–1013) | >.1 # | ||||||
| Clinician assessment of ICB nerve damage, No. (%) | |||||||||||||||
| None | 351 (86) | 343 (86) | 350 (85) | 365 (91) | 231 (59) | 233 (62) | 251 (64) | 264 (69) | <.001 ** | ||||||
| Mild | 52 (13) | 50 (13) | 56 (14) | 30 (8) | 151 (39) | 130 (35) | 133 (34) | 115 (30) | |||||||
| Severe | 6 (1) | 4 (1) | 4 (1) | 5 (1) | 10 (3) | 10 (3) | 10 (3) | 5 (1) | |||||||
| Mean change in shoulder function, degrees (95% CI) †† | |||||||||||||||
| Flexion | 5.8 (4.3 to 7.4) | 2.0 (0.9 to 3.2) | 2.0 (0.7 to 3.3) | 2.7 (1.2 to 4.2) | 9.8 (7.9 to 11.7) | 3.7 (2.0 to 5.4) | 1.6 (0.2 to 3.0) | 0.1 (−1.2 to 1.5) | .004 ‡‡ | ||||||
| Abduction | 6.5 (4.5 to 8.6) | 1.9 (0.5 to 3.4) | 1.5 (−0.1 to 3.1) | 2.5 (0.6 to 4.4) | 12.9 (10.7 to 15.2) | 4.2 (2.4 to 6.1) | 2.3 (0.7 to 4.0) | 1.9 (0.2 to 3.5) | .001 ‡‡ | ||||||
| External rotation | 0.7 (−0.4 to 1.8) | 0.2 (−0.9 to 1.3) | 0.6 (−0.7 to 1.9) | 0.6 (−0.8 to 2.0) | 1.2 (0.2 to 2.3) | 1.2 (0.0 to 2.3) | 1.0 (−0.2 to 2.1) | 0.7 (−0.8 to 2.1) | .18 ‡‡ | ||||||
| Internal rotation | 0.4 (−0.9 to 1.6) | 1.0 (−0.2 to 2.3) | 0.2 (−1.2 to 1.5) | 1.7 (0.4 to 3.0) | 0.9 (−0.4 to 2.1) | 0.7 (−0.5 to 2.0) | 0.8 (−0.6 to 2.1) | 0.4 (−1.0 to 1.8) | .31 ‡‡ | ||||||
ICB = intercostobrachial; CI = confidence interval.
Two-sided chi-square test for trend, with 1 degree of freedom, comparing the three-category ordinal lymphedema self-assessment variable between sentinel lymph nose biopsy and standard treatment groups give P <.001 at each of the four follow-up times.
Mean change in ipsilateral arm volume at each follow-up visit expressed as the ratio compared with the pretreatment value.
Two-sided t test comparing ipsicontralateral differences in change in log volume between sentinel lymph node biopsy group and standard treatment group give P <001, P = .001, P = .005, and P = .096 at 1, 3, 6, and 12 months, respectively.
Chi-square test with 1 degree of freedom, comparing incidence of self-assessed sensory loss between sentinel lymph node biopsy group and standard treatment group give P <.001 at each of the four follow-up times. Corresponding numbers needed to treat to obtain benefit are 2.3, 2.9, 3.7, and 5.1, respectively.
Total area of sensory loss for the axilla and the upper arm among patients who reported sensory loss as determined by the pin-prick method.
Mann–Whitney U tests comparing area of sensory loss between sentinel lymph node biopsy group and standard treatment group give P = .72, P = .70, P = .63 and P = .37 at the four follow-up times.
Two-sided chi-square test for trend, with 1 degree of freedom, comparing the three-category ordinal ICB nerve damage variable between sentinel lymph node biopsy and standard treatment groups give P <.001 at each of the four follow-up times.
Ipsilateral shoulder function impairment at each follow-up visit calculated by subtracting from the pretreatment range of movement.
t tests comparing ipsicontralateral differences in change in flexion, abduction, external rotation, and internal rotation between sentinal lymph node biopsy group and standard treatment group at 1, 3, 6, and 12 months give P = .004, P = .33, P = .98, and P = .054, respectively (flexion); P = .001, P = .14, P = .60, and P = .27, respectively (abduction); P = .18, P = .93, P = .16, and P = .89, respectively (external rotation); and P = .31, P = .73, P = .94, and P = .39, respectively (internal rotation).
Postoperative arm morbidity after sentinel node biopsy and standard axillary treatment in the ALMANAC trial *
| Arm morbidity assessment . | Sentinel lymph node biopsy (n = 478 ) Time after surgery (mo) . | . | . | . | Standard axillary treatment (n = 476) Time after surgery (mo) . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 3 . | 6 . | 12 . | 1 . | 3 . | 6 . | 12 . | P . | ||||||
| Lymphedema | |||||||||||||||
| Self-assessment, No. (%) | |||||||||||||||
| None | 414 (97) | 397 (95) | 413 (96) | 392 (95) | 369 (88) | 334 (85) | 343 (83) | 350 (87) | |||||||
| Mild | 13 (3) | 16 (4) | 17 (4) | 16 (4) | 43 (10) | 49 (12) | 58 (14) | 43 (11) | |||||||
| Moderate or severe | 1 ( 0.2) | 4 (1) | 2 (0.5) | 4 (1) | 7 (2) | 12 (3) | 13 (3) | 10 (2) | <.001 † | ||||||
| Mean change in arm volume (95% CI) ‡ | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | <.005 § | ||||||
| Sensory deficit | |||||||||||||||
| Self-assessment, No. (%) | 74/411 (18) | 81/407 (20) | 69/423 (16) | 46/407 (11) | 252/409 (62) | 209/388 (54) | 178/411 (43) | 124/401 (31) | <.001 ‖ | ||||||
| Median area of sensory loss, cm 2 (range) ¶ | 32 (2–254) | 48 (0–327) | 32 (0–201) | 59 (0.2–342) | 40 (1–489) | 47 (0–1139) | 39 (0.4–2827) | 35 (0.8–1013) | >.1 # | ||||||
| Clinician assessment of ICB nerve damage, No. (%) | |||||||||||||||
| None | 351 (86) | 343 (86) | 350 (85) | 365 (91) | 231 (59) | 233 (62) | 251 (64) | 264 (69) | <.001 ** | ||||||
| Mild | 52 (13) | 50 (13) | 56 (14) | 30 (8) | 151 (39) | 130 (35) | 133 (34) | 115 (30) | |||||||
| Severe | 6 (1) | 4 (1) | 4 (1) | 5 (1) | 10 (3) | 10 (3) | 10 (3) | 5 (1) | |||||||
| Mean change in shoulder function, degrees (95% CI) †† | |||||||||||||||
| Flexion | 5.8 (4.3 to 7.4) | 2.0 (0.9 to 3.2) | 2.0 (0.7 to 3.3) | 2.7 (1.2 to 4.2) | 9.8 (7.9 to 11.7) | 3.7 (2.0 to 5.4) | 1.6 (0.2 to 3.0) | 0.1 (−1.2 to 1.5) | .004 ‡‡ | ||||||
| Abduction | 6.5 (4.5 to 8.6) | 1.9 (0.5 to 3.4) | 1.5 (−0.1 to 3.1) | 2.5 (0.6 to 4.4) | 12.9 (10.7 to 15.2) | 4.2 (2.4 to 6.1) | 2.3 (0.7 to 4.0) | 1.9 (0.2 to 3.5) | .001 ‡‡ | ||||||
| External rotation | 0.7 (−0.4 to 1.8) | 0.2 (−0.9 to 1.3) | 0.6 (−0.7 to 1.9) | 0.6 (−0.8 to 2.0) | 1.2 (0.2 to 2.3) | 1.2 (0.0 to 2.3) | 1.0 (−0.2 to 2.1) | 0.7 (−0.8 to 2.1) | .18 ‡‡ | ||||||
| Internal rotation | 0.4 (−0.9 to 1.6) | 1.0 (−0.2 to 2.3) | 0.2 (−1.2 to 1.5) | 1.7 (0.4 to 3.0) | 0.9 (−0.4 to 2.1) | 0.7 (−0.5 to 2.0) | 0.8 (−0.6 to 2.1) | 0.4 (−1.0 to 1.8) | .31 ‡‡ | ||||||
| Arm morbidity assessment . | Sentinel lymph node biopsy (n = 478 ) Time after surgery (mo) . | . | . | . | Standard axillary treatment (n = 476) Time after surgery (mo) . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 3 . | 6 . | 12 . | 1 . | 3 . | 6 . | 12 . | P . | ||||||
| Lymphedema | |||||||||||||||
| Self-assessment, No. (%) | |||||||||||||||
| None | 414 (97) | 397 (95) | 413 (96) | 392 (95) | 369 (88) | 334 (85) | 343 (83) | 350 (87) | |||||||
| Mild | 13 (3) | 16 (4) | 17 (4) | 16 (4) | 43 (10) | 49 (12) | 58 (14) | 43 (11) | |||||||
| Moderate or severe | 1 ( 0.2) | 4 (1) | 2 (0.5) | 4 (1) | 7 (2) | 12 (3) | 13 (3) | 10 (2) | <.001 † | ||||||
| Mean change in arm volume (95% CI) ‡ | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | 1.003 (0.997 to 1.010) | 1.019 (1.010 to 1.028) | 1.022 (1.011 to 1.032) | 1.028 (1.016 to 1.039) | <.005 § | ||||||
| Sensory deficit | |||||||||||||||
| Self-assessment, No. (%) | 74/411 (18) | 81/407 (20) | 69/423 (16) | 46/407 (11) | 252/409 (62) | 209/388 (54) | 178/411 (43) | 124/401 (31) | <.001 ‖ | ||||||
| Median area of sensory loss, cm 2 (range) ¶ | 32 (2–254) | 48 (0–327) | 32 (0–201) | 59 (0.2–342) | 40 (1–489) | 47 (0–1139) | 39 (0.4–2827) | 35 (0.8–1013) | >.1 # | ||||||
| Clinician assessment of ICB nerve damage, No. (%) | |||||||||||||||
| None | 351 (86) | 343 (86) | 350 (85) | 365 (91) | 231 (59) | 233 (62) | 251 (64) | 264 (69) | <.001 ** | ||||||
| Mild | 52 (13) | 50 (13) | 56 (14) | 30 (8) | 151 (39) | 130 (35) | 133 (34) | 115 (30) | |||||||
| Severe | 6 (1) | 4 (1) | 4 (1) | 5 (1) | 10 (3) | 10 (3) | 10 (3) | 5 (1) | |||||||
| Mean change in shoulder function, degrees (95% CI) †† | |||||||||||||||
| Flexion | 5.8 (4.3 to 7.4) | 2.0 (0.9 to 3.2) | 2.0 (0.7 to 3.3) | 2.7 (1.2 to 4.2) | 9.8 (7.9 to 11.7) | 3.7 (2.0 to 5.4) | 1.6 (0.2 to 3.0) | 0.1 (−1.2 to 1.5) | .004 ‡‡ | ||||||
| Abduction | 6.5 (4.5 to 8.6) | 1.9 (0.5 to 3.4) | 1.5 (−0.1 to 3.1) | 2.5 (0.6 to 4.4) | 12.9 (10.7 to 15.2) | 4.2 (2.4 to 6.1) | 2.3 (0.7 to 4.0) | 1.9 (0.2 to 3.5) | .001 ‡‡ | ||||||
| External rotation | 0.7 (−0.4 to 1.8) | 0.2 (−0.9 to 1.3) | 0.6 (−0.7 to 1.9) | 0.6 (−0.8 to 2.0) | 1.2 (0.2 to 2.3) | 1.2 (0.0 to 2.3) | 1.0 (−0.2 to 2.1) | 0.7 (−0.8 to 2.1) | .18 ‡‡ | ||||||
| Internal rotation | 0.4 (−0.9 to 1.6) | 1.0 (−0.2 to 2.3) | 0.2 (−1.2 to 1.5) | 1.7 (0.4 to 3.0) | 0.9 (−0.4 to 2.1) | 0.7 (−0.5 to 2.0) | 0.8 (−0.6 to 2.1) | 0.4 (−1.0 to 1.8) | .31 ‡‡ | ||||||
ICB = intercostobrachial; CI = confidence interval.
Two-sided chi-square test for trend, with 1 degree of freedom, comparing the three-category ordinal lymphedema self-assessment variable between sentinel lymph nose biopsy and standard treatment groups give P <.001 at each of the four follow-up times.
Mean change in ipsilateral arm volume at each follow-up visit expressed as the ratio compared with the pretreatment value.
Two-sided t test comparing ipsicontralateral differences in change in log volume between sentinel lymph node biopsy group and standard treatment group give P <001, P = .001, P = .005, and P = .096 at 1, 3, 6, and 12 months, respectively.
Chi-square test with 1 degree of freedom, comparing incidence of self-assessed sensory loss between sentinel lymph node biopsy group and standard treatment group give P <.001 at each of the four follow-up times. Corresponding numbers needed to treat to obtain benefit are 2.3, 2.9, 3.7, and 5.1, respectively.
Total area of sensory loss for the axilla and the upper arm among patients who reported sensory loss as determined by the pin-prick method.
Mann–Whitney U tests comparing area of sensory loss between sentinel lymph node biopsy group and standard treatment group give P = .72, P = .70, P = .63 and P = .37 at the four follow-up times.
Two-sided chi-square test for trend, with 1 degree of freedom, comparing the three-category ordinal ICB nerve damage variable between sentinel lymph node biopsy and standard treatment groups give P <.001 at each of the four follow-up times.
Ipsilateral shoulder function impairment at each follow-up visit calculated by subtracting from the pretreatment range of movement.
t tests comparing ipsicontralateral differences in change in flexion, abduction, external rotation, and internal rotation between sentinal lymph node biopsy group and standard treatment group at 1, 3, 6, and 12 months give P = .004, P = .33, P = .98, and P = .054, respectively (flexion); P = .001, P = .14, P = .60, and P = .27, respectively (abduction); P = .18, P = .93, P = .16, and P = .89, respectively (external rotation); and P = .31, P = .73, P = .94, and P = .39, respectively (internal rotation).
Other efficacy assessments in the ALMANAC trial *
| Efficacy endpoint . | Sentinel lymph node biopsy (n = 478 ) . | Standard axillary treatment (n = 476) . | P† . |
|---|---|---|---|
| Median axillary operative time, min (IQR) | 17 (12–29) | 20 (13–30) | .055 |
| Axillary drain usage ‡ , No. (%) | 75 (17) | 359 (79) | <.001 § |
| Mean hospital stay, days (95% CI) | 4.1 (3.8 to 4.5) | 5.4 (5.1 to 5.6) | <.001 |
| No. patients with an infection (%) | 52 (11) | 72 (15) | .051 § |
| Time from surgery to return to normal day-to-day activities ‖ , No. (%) | |||
| 1 wk | 131 (31) | 98 (23) | <.001 |
| 2 wks | 234 (55) | 180 (42) | |
| 3 wks | 286 (67) | 235 (55) | |
| 1 mo | 329 (77) | 308 (72) | |
| 3 mo | 400 (94) | 389 (91) | |
| 6 mo | 413 (97) | 406 (95) | |
| 12 mo | 414 (97) | 410 (96) | |
| Time from surgery to return to paid work ¶ , No. (%) | |||
| 1 mo | 54 (29) | 52 (26) | .21 |
| 3 mo | 90 (49) | 95 (48) | |
| 6 mo | 110 (60) | 111 (56) | |
| 12 mo | 145 (79) | 142 (71) |
| Efficacy endpoint . | Sentinel lymph node biopsy (n = 478 ) . | Standard axillary treatment (n = 476) . | P† . |
|---|---|---|---|
| Median axillary operative time, min (IQR) | 17 (12–29) | 20 (13–30) | .055 |
| Axillary drain usage ‡ , No. (%) | 75 (17) | 359 (79) | <.001 § |
| Mean hospital stay, days (95% CI) | 4.1 (3.8 to 4.5) | 5.4 (5.1 to 5.6) | <.001 |
| No. patients with an infection (%) | 52 (11) | 72 (15) | .051 § |
| Time from surgery to return to normal day-to-day activities ‖ , No. (%) | |||
| 1 wk | 131 (31) | 98 (23) | <.001 |
| 2 wks | 234 (55) | 180 (42) | |
| 3 wks | 286 (67) | 235 (55) | |
| 1 mo | 329 (77) | 308 (72) | |
| 3 mo | 400 (94) | 389 (91) | |
| 6 mo | 413 (97) | 406 (95) | |
| 12 mo | 414 (97) | 410 (96) | |
| Time from surgery to return to paid work ¶ , No. (%) | |||
| 1 mo | 54 (29) | 52 (26) | .21 |
| 3 mo | 90 (49) | 95 (48) | |
| 6 mo | 110 (60) | 111 (56) | |
| 12 mo | 145 (79) | 142 (71) |
IQR = interquartile range; CI = confidence interval.
Mann–Whitney U test (two-sided).
Denominators: 449 (sentinel lymph node biopsy group) and 453 (standard axillary treatment group).
Two-sided chi-square test.
Based on 425 patients in sentinel lymph node biopsy group and 426 patients in standard axillary treatment group.
Based on 184 patients in sentinel lymph node biopsy group and 199 patients in standard axillary treatment group; 230 of 414 patients in the sentinel node group and 219 of 418 patients in the standard axillary treatment group with adequate data did not undertake paid work before treatment.
Other efficacy assessments in the ALMANAC trial *
| Efficacy endpoint . | Sentinel lymph node biopsy (n = 478 ) . | Standard axillary treatment (n = 476) . | P† . |
|---|---|---|---|
| Median axillary operative time, min (IQR) | 17 (12–29) | 20 (13–30) | .055 |
| Axillary drain usage ‡ , No. (%) | 75 (17) | 359 (79) | <.001 § |
| Mean hospital stay, days (95% CI) | 4.1 (3.8 to 4.5) | 5.4 (5.1 to 5.6) | <.001 |
| No. patients with an infection (%) | 52 (11) | 72 (15) | .051 § |
| Time from surgery to return to normal day-to-day activities ‖ , No. (%) | |||
| 1 wk | 131 (31) | 98 (23) | <.001 |
| 2 wks | 234 (55) | 180 (42) | |
| 3 wks | 286 (67) | 235 (55) | |
| 1 mo | 329 (77) | 308 (72) | |
| 3 mo | 400 (94) | 389 (91) | |
| 6 mo | 413 (97) | 406 (95) | |
| 12 mo | 414 (97) | 410 (96) | |
| Time from surgery to return to paid work ¶ , No. (%) | |||
| 1 mo | 54 (29) | 52 (26) | .21 |
| 3 mo | 90 (49) | 95 (48) | |
| 6 mo | 110 (60) | 111 (56) | |
| 12 mo | 145 (79) | 142 (71) |
| Efficacy endpoint . | Sentinel lymph node biopsy (n = 478 ) . | Standard axillary treatment (n = 476) . | P† . |
|---|---|---|---|
| Median axillary operative time, min (IQR) | 17 (12–29) | 20 (13–30) | .055 |
| Axillary drain usage ‡ , No. (%) | 75 (17) | 359 (79) | <.001 § |
| Mean hospital stay, days (95% CI) | 4.1 (3.8 to 4.5) | 5.4 (5.1 to 5.6) | <.001 |
| No. patients with an infection (%) | 52 (11) | 72 (15) | .051 § |
| Time from surgery to return to normal day-to-day activities ‖ , No. (%) | |||
| 1 wk | 131 (31) | 98 (23) | <.001 |
| 2 wks | 234 (55) | 180 (42) | |
| 3 wks | 286 (67) | 235 (55) | |
| 1 mo | 329 (77) | 308 (72) | |
| 3 mo | 400 (94) | 389 (91) | |
| 6 mo | 413 (97) | 406 (95) | |
| 12 mo | 414 (97) | 410 (96) | |
| Time from surgery to return to paid work ¶ , No. (%) | |||
| 1 mo | 54 (29) | 52 (26) | .21 |
| 3 mo | 90 (49) | 95 (48) | |
| 6 mo | 110 (60) | 111 (56) | |
| 12 mo | 145 (79) | 142 (71) |
IQR = interquartile range; CI = confidence interval.
Mann–Whitney U test (two-sided).
Denominators: 449 (sentinel lymph node biopsy group) and 453 (standard axillary treatment group).
Two-sided chi-square test.
Based on 425 patients in sentinel lymph node biopsy group and 426 patients in standard axillary treatment group.
Based on 184 patients in sentinel lymph node biopsy group and 199 patients in standard axillary treatment group; 230 of 414 patients in the sentinel node group and 219 of 418 patients in the standard axillary treatment group with adequate data did not undertake paid work before treatment.
Sentinel Node Biopsy and Perioperative Findings
The failed sentinel lymph node localization rate was 2.0% (95% CI = 1.1% to 3.7%). Drainage of isotope to axillary lymph nodes was visualized by lymphoscintigraphy in 341 (73%) of 468 patients. Of the 468 patients who underwent lymphoscintigraphy, 44 were recorded as showing drainage of isotope to internal mammary lymph nodes. Another seven patients had lymphatic drainage to the internal mammary chain that could only be detected intraoperatively by the gamma probe. The median number of sentinel lymph nodes removed per patient was 2 (range = 1–11). The prevalence of axillary nodal metastases ( Table 1 ) was slightly higher in patients treated by sentinel lymph node biopsy than in those treated by standard axillary treatment (26% versus 23%; difference = 2.4%, 95% CI = −3.0% to 7.7%), suggesting that the number of positive nodes missed by sentinel lymph node biopsy (i.e., the false-negative rate) was likely to have been small.
Of the 121 patients who had sentinel lymph node metastases and for whom axillary treatment information was available, 83 patients proceeded to delayed completion axillary lymph node dissection (primary surgery was wide local excision in 70 patients and mastectomy in 13 patients), 33 patients received axillary radiotherapy (primary surgery wide local excision in 28 patients and mastectomy in five patients), and five patients chose to have no further treatment. A total of 51 patients had drainage to the internal mammary chain (44 patients had drainage detected during preoperative lymphoscintigraphy and seven patients had drainage detected by the gamma probe during surgery); of those 51 patients, 29 had sampling of nodes in the internal mammary chain and four were found to have internal mammary metastases. Three of these four patients had metastatic involvement in the internal mammary nodes without apparent accompanying axillary metastases. However, two of the three patients with metastatic involvement in the internal mammary nodes did not have axillary drainage on lymphoscintigraphy or gamma probe detection; because both patients underwent internal mammary sentinel lymph node biopsy only, the final axillary histology is not known. The true rate of internal mammary node positivity in the absence of axillary node metastases is therefore unclear, but it is likely to be less than 1% ( 32 ) .
Of the 496 patients assigned to standard axillary treatment, 123 patients (25%) underwent four-node sampling, with a median of five nodes (range = 2–25 nodes) removed per patient. The remaining 373 patients underwent axillary lymph node dissection, with a median of 15 nodes (range = 1–42 nodes) removed per patient.
Postoperative Arm Morbidity After Surgery
Lymphedema. Moderate or severe lymphedema was reported more often by patients in the standard axillary treatment group than by patients in the sentinel lymph node biopsy group at 1, 3, 6, and 12 months after surgery (e.g., 5% versus 13% at 12 months; all P <.001) ( Table 2 ). The relative risk of any lymphedema for the sentinel lymph node biopsy group compared with that of the standard axillary treatment group at 12 months was 0.37 (95% CI = 0.23 to 0.60).
Analyses of circumferential measurements of the upper arm and forearm indicated that patients in the standard axillary treatment group had statistically significantly more arm swelling at 1, 3, and 6 months after surgery than patients in the standard axillary treatment group ( P <.001, P = .001, and P = .003 respectively; e.g., ratio of arm volume at 6 months to arm volume at baseline: 1.02 in the sentinel lymph node biopsy group versus 1.06 in the standard treatment group). At 12 months after surgery, although the sentinel lymph node biopsy group had less arm swelling than the standard axillary treatment group, the difference was not statistically significant.
Sensory deficit. Sensory loss at 1 month after surgery was reported by 18% of patients in the sentinel lymph node biopsy group and by 62% of patients in the standard axillary treatment group. At 12 months after surgery, the percentage of patients reporting sensory loss had declined to 11% in the sentinel lymph node biopsy group and to 31% in the standard axillary treatment group; at all time points, statistically significantly more patients in the standard treatment group than in the sentinel biopsy groups reported sensory deficit ( P <.001 for all; Table 2 ). The relative risk of sensory deficit at 12 months was 0.37 (95% CI = 0.27 to 0.50) in favor of the sentinel lymph node biopsy group. Among the patients who reported sensory deficit, the extent of sensory loss, as determined by the pin-prick method, was similar in the two groups ( Table 2 ). Clinician-assessed intercostobrachial nerve damage was more extensive in the standard treatment group than in the sentinel lymph node biopsy group at 1, 3, 6, and 12 months after surgery (all P <.001; Table 2 ).
Shoulder function. Compared with patients in the sentinel node biopsy group, those in the standard axillary treatment group displayed statistically significantly more impairment of shoulder flexion and abduction on the ipsilateral side at 1 month after surgery ( P = .004 and .001, respectively). However, shoulder flexion and abduction improved rapidly at the subsequent time points in both groups, and differences between the groups were no longer statistically significant. There was no statistically significant difference in shoulder internal or external rotation between the two groups at any time point.
Other Efficacy Assessments
Table 3 summarizes the other prespecified trial endpoints. The median duration of axillary surgery was 23.4 minutes (interquartile range [IQR] = 13–30 minutes) for patients in the standard treatment arm and 23.3 minutes (IQR = 12–29 minutes) for patients in the sentinel lymph node biopsy arm ( P = .055). Patients in the sentinel node biopsy group had a statistically significantly shorter mean hospital stay than patients in the standard treatment group (4.1 days versus 5.4 days; difference = 1.2 days, 95% CI = 0.8 to 1.6 days, P <.001). Fewer patients in the sentinel lymph node biopsy group than in the standard treatment group had axillary drain usage (17% versus 79%; difference = 63%, 95% CI = 57% to 67%, number needed to treat to obtain benefit [NNT] 1.6, P <.001). The incidence of infection was 4% lower (95% CI = 0% to 8%, NNT 24) in the sentinel lymph node biopsy group (11%) than in standard axillary treatment group (15%) ( P = .051). Patients allocated to sentinel lymph node biopsy resumed their normal day-to-day activities sooner after surgery than patients allocated to standard treatment ( P <.001), but there was no statistically significant difference between the two groups in the length of time until they returned to paid work.
Quality-of-Life Assessments
TOI score. Baseline scores were compared with later scores to examine the change in TOI over time. There were statistically significant differences in TOI scores between treatment groups favoring the sentinel lymph node biopsy group at all time points ( P <.001, 1 month after surgery; P = .001, 3, 6, and 12 months after surgery). For both groups, mean TOI scores were lowest at 1 month after surgery and then improved over time at the later follow-ups ( Table 4 ).
Summary statistics for quality-of-life scales by time and treatment group *
| Quality-of-life scores . | Baseline . | 1 mo . | 3 mo . | 6 mo . | 12 mo . |
|---|---|---|---|---|---|
| Trial outcome index, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 86.7 (85.6 to 87.9) | 82.9 (81.3 to 84.4) | 84.4 (82.8 to 86.0) | 84.9 (83.4 to 86.5) | 89.2 (87.9 to 90.4) |
| Standard axillary treatment | 87.7 (86.6 to 88.9) | 78.7 (77.1 to 80.3) | 81.9 (80.3 to 83.5) | 82.4 (80.8 to 84.1) | 87.1 (85.6 to 88.6) |
| P† | <.001 | .001 | .001 | .001 | |
| Trial outcome index reduced by ≥5 points from baseline, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 154/371 (42) | 130/371 (35) | 134/369 (36) | 84/363 (23) | |
| Standard axillary treatment | 206/359 (57) | 172/356 (48) | 168/354 (47) | 120/344 (35) | |
| NNT | 6.3 | 7.5 | 9.0 | 8.5 | |
| P‡ | <.001 | <.001 | .002 | .001 | |
| Arm functioning subscale score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 19.2 (19.0 to 19.4) | 17.6 (17.3 to 18.0) | 18.5 (18.3 to 18.7) | 18.3 (18.1 to 18.6) | 18.5 (18.2 to 18.7) |
| Standard axillary treatment | 19.2 (19.0 to 19.5) | 14.6 (14.2 to 15.0) | 16.7 (16.4 to 17.1) | 16.8 (16.5 to 17.2) | 17.1 (16.8 to 17.5) |
| P† | <.001 | <.001 | <.001 | <.001 | |
| Substantial arm swelling or tenderness, No/total No. (%) | |||||
| Sentinel lymph node biopsy | 15/412 (4) | 47/388 (12) | 22/389 (6) | 29/387 (7) | 20/381(5) |
| Standard axillary treatment | 17/387 (4) | 106/379 (28) | 55/379 (15) | 59/374 (16) | 42/362 (12) |
| NNT | 6.3 | 11.3 | 12.1 | 15.7 | |
| P‡ | <.001 | <.001 | <.001 | .002 | |
| Substantial numbness on ipsilateral side, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 4/408 (1) | 36/388 (9) | 30/391 (8) | 35/389 (9) | 23/386 (6) |
| Standard axillary treatment | 10/385 (3) | 113/381 (30) | 101/380 (27) | 100/377 (27) | 74/363 (20) |
| NNT | 4.9 | 5.3 | 5.7 | 6.9 | |
| P‡ | <.001 | <.001 | <.001 | <.001 | |
| FACT-B+4 score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 127.5 (125.8 to 129.2) | 126.0 (123.9 to 128.1) | 128.0 (125.9 to 130.1) | 127.9 (125.7 to 130.0) | 132.7 (130.9 to 134.6) |
| Standard axillary treatment | 129.1 (127.4 to 130.8) | 121.7 (119.6 to 123.8) | 125.2 (123.0 to 127.3) | 125.4 (123.1 to 127.6) | 130.5 (128.4 to 132.6) |
| P† | <.001 | .001 | .003 | .002 | |
| STAI score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 43.4 (42.1 to 44.7) | 37.4 (36.1 to 38.7) | 35.9 (34.7 to 37.2) | 36.0 (34.7 to 37.2) | 33.7 (32.5 to 35.0) |
| Standard axillary treatment | 42.9 (41.5 to 44.2) | 37.4 (36.1 to 38.6) | 36.3 (35.1 to 37.5) | 36.1 (34.8 to 37.4) | 34.2 (32.9 to 35.4) |
| P§ | .77 | .37 | .61 | .35 |
| Quality-of-life scores . | Baseline . | 1 mo . | 3 mo . | 6 mo . | 12 mo . |
|---|---|---|---|---|---|
| Trial outcome index, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 86.7 (85.6 to 87.9) | 82.9 (81.3 to 84.4) | 84.4 (82.8 to 86.0) | 84.9 (83.4 to 86.5) | 89.2 (87.9 to 90.4) |
| Standard axillary treatment | 87.7 (86.6 to 88.9) | 78.7 (77.1 to 80.3) | 81.9 (80.3 to 83.5) | 82.4 (80.8 to 84.1) | 87.1 (85.6 to 88.6) |
| P† | <.001 | .001 | .001 | .001 | |
| Trial outcome index reduced by ≥5 points from baseline, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 154/371 (42) | 130/371 (35) | 134/369 (36) | 84/363 (23) | |
| Standard axillary treatment | 206/359 (57) | 172/356 (48) | 168/354 (47) | 120/344 (35) | |
| NNT | 6.3 | 7.5 | 9.0 | 8.5 | |
| P‡ | <.001 | <.001 | .002 | .001 | |
| Arm functioning subscale score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 19.2 (19.0 to 19.4) | 17.6 (17.3 to 18.0) | 18.5 (18.3 to 18.7) | 18.3 (18.1 to 18.6) | 18.5 (18.2 to 18.7) |
| Standard axillary treatment | 19.2 (19.0 to 19.5) | 14.6 (14.2 to 15.0) | 16.7 (16.4 to 17.1) | 16.8 (16.5 to 17.2) | 17.1 (16.8 to 17.5) |
| P† | <.001 | <.001 | <.001 | <.001 | |
| Substantial arm swelling or tenderness, No/total No. (%) | |||||
| Sentinel lymph node biopsy | 15/412 (4) | 47/388 (12) | 22/389 (6) | 29/387 (7) | 20/381(5) |
| Standard axillary treatment | 17/387 (4) | 106/379 (28) | 55/379 (15) | 59/374 (16) | 42/362 (12) |
| NNT | 6.3 | 11.3 | 12.1 | 15.7 | |
| P‡ | <.001 | <.001 | <.001 | .002 | |
| Substantial numbness on ipsilateral side, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 4/408 (1) | 36/388 (9) | 30/391 (8) | 35/389 (9) | 23/386 (6) |
| Standard axillary treatment | 10/385 (3) | 113/381 (30) | 101/380 (27) | 100/377 (27) | 74/363 (20) |
| NNT | 4.9 | 5.3 | 5.7 | 6.9 | |
| P‡ | <.001 | <.001 | <.001 | <.001 | |
| FACT-B+4 score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 127.5 (125.8 to 129.2) | 126.0 (123.9 to 128.1) | 128.0 (125.9 to 130.1) | 127.9 (125.7 to 130.0) | 132.7 (130.9 to 134.6) |
| Standard axillary treatment | 129.1 (127.4 to 130.8) | 121.7 (119.6 to 123.8) | 125.2 (123.0 to 127.3) | 125.4 (123.1 to 127.6) | 130.5 (128.4 to 132.6) |
| P† | <.001 | .001 | .003 | .002 | |
| STAI score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 43.4 (42.1 to 44.7) | 37.4 (36.1 to 38.7) | 35.9 (34.7 to 37.2) | 36.0 (34.7 to 37.2) | 33.7 (32.5 to 35.0) |
| Standard axillary treatment | 42.9 (41.5 to 44.2) | 37.4 (36.1 to 38.6) | 36.3 (35.1 to 37.5) | 36.1 (34.8 to 37.4) | 34.2 (32.9 to 35.4) |
| P§ | .77 | .37 | .61 | .35 |
Based on up to 424 patients allocated to sentinel lymph node biopsy and up to 405 patients allocated to standard management. CI = confidence interval; FACT-B+4 = Functional Assessment of Cancer Therapy-Breast +4 questionnaire; STAI = Spielberger State/Trait Anxiety Inventory; NNT = number needed to treat to obtain benefit.
Two-sided Student's t test comparing changes from baseline between groups.
Two-sided chi-square test.
Two-sided analysis of covariance.
Summary statistics for quality-of-life scales by time and treatment group *
| Quality-of-life scores . | Baseline . | 1 mo . | 3 mo . | 6 mo . | 12 mo . |
|---|---|---|---|---|---|
| Trial outcome index, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 86.7 (85.6 to 87.9) | 82.9 (81.3 to 84.4) | 84.4 (82.8 to 86.0) | 84.9 (83.4 to 86.5) | 89.2 (87.9 to 90.4) |
| Standard axillary treatment | 87.7 (86.6 to 88.9) | 78.7 (77.1 to 80.3) | 81.9 (80.3 to 83.5) | 82.4 (80.8 to 84.1) | 87.1 (85.6 to 88.6) |
| P† | <.001 | .001 | .001 | .001 | |
| Trial outcome index reduced by ≥5 points from baseline, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 154/371 (42) | 130/371 (35) | 134/369 (36) | 84/363 (23) | |
| Standard axillary treatment | 206/359 (57) | 172/356 (48) | 168/354 (47) | 120/344 (35) | |
| NNT | 6.3 | 7.5 | 9.0 | 8.5 | |
| P‡ | <.001 | <.001 | .002 | .001 | |
| Arm functioning subscale score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 19.2 (19.0 to 19.4) | 17.6 (17.3 to 18.0) | 18.5 (18.3 to 18.7) | 18.3 (18.1 to 18.6) | 18.5 (18.2 to 18.7) |
| Standard axillary treatment | 19.2 (19.0 to 19.5) | 14.6 (14.2 to 15.0) | 16.7 (16.4 to 17.1) | 16.8 (16.5 to 17.2) | 17.1 (16.8 to 17.5) |
| P† | <.001 | <.001 | <.001 | <.001 | |
| Substantial arm swelling or tenderness, No/total No. (%) | |||||
| Sentinel lymph node biopsy | 15/412 (4) | 47/388 (12) | 22/389 (6) | 29/387 (7) | 20/381(5) |
| Standard axillary treatment | 17/387 (4) | 106/379 (28) | 55/379 (15) | 59/374 (16) | 42/362 (12) |
| NNT | 6.3 | 11.3 | 12.1 | 15.7 | |
| P‡ | <.001 | <.001 | <.001 | .002 | |
| Substantial numbness on ipsilateral side, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 4/408 (1) | 36/388 (9) | 30/391 (8) | 35/389 (9) | 23/386 (6) |
| Standard axillary treatment | 10/385 (3) | 113/381 (30) | 101/380 (27) | 100/377 (27) | 74/363 (20) |
| NNT | 4.9 | 5.3 | 5.7 | 6.9 | |
| P‡ | <.001 | <.001 | <.001 | <.001 | |
| FACT-B+4 score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 127.5 (125.8 to 129.2) | 126.0 (123.9 to 128.1) | 128.0 (125.9 to 130.1) | 127.9 (125.7 to 130.0) | 132.7 (130.9 to 134.6) |
| Standard axillary treatment | 129.1 (127.4 to 130.8) | 121.7 (119.6 to 123.8) | 125.2 (123.0 to 127.3) | 125.4 (123.1 to 127.6) | 130.5 (128.4 to 132.6) |
| P† | <.001 | .001 | .003 | .002 | |
| STAI score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 43.4 (42.1 to 44.7) | 37.4 (36.1 to 38.7) | 35.9 (34.7 to 37.2) | 36.0 (34.7 to 37.2) | 33.7 (32.5 to 35.0) |
| Standard axillary treatment | 42.9 (41.5 to 44.2) | 37.4 (36.1 to 38.6) | 36.3 (35.1 to 37.5) | 36.1 (34.8 to 37.4) | 34.2 (32.9 to 35.4) |
| P§ | .77 | .37 | .61 | .35 |
| Quality-of-life scores . | Baseline . | 1 mo . | 3 mo . | 6 mo . | 12 mo . |
|---|---|---|---|---|---|
| Trial outcome index, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 86.7 (85.6 to 87.9) | 82.9 (81.3 to 84.4) | 84.4 (82.8 to 86.0) | 84.9 (83.4 to 86.5) | 89.2 (87.9 to 90.4) |
| Standard axillary treatment | 87.7 (86.6 to 88.9) | 78.7 (77.1 to 80.3) | 81.9 (80.3 to 83.5) | 82.4 (80.8 to 84.1) | 87.1 (85.6 to 88.6) |
| P† | <.001 | .001 | .001 | .001 | |
| Trial outcome index reduced by ≥5 points from baseline, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 154/371 (42) | 130/371 (35) | 134/369 (36) | 84/363 (23) | |
| Standard axillary treatment | 206/359 (57) | 172/356 (48) | 168/354 (47) | 120/344 (35) | |
| NNT | 6.3 | 7.5 | 9.0 | 8.5 | |
| P‡ | <.001 | <.001 | .002 | .001 | |
| Arm functioning subscale score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 19.2 (19.0 to 19.4) | 17.6 (17.3 to 18.0) | 18.5 (18.3 to 18.7) | 18.3 (18.1 to 18.6) | 18.5 (18.2 to 18.7) |
| Standard axillary treatment | 19.2 (19.0 to 19.5) | 14.6 (14.2 to 15.0) | 16.7 (16.4 to 17.1) | 16.8 (16.5 to 17.2) | 17.1 (16.8 to 17.5) |
| P† | <.001 | <.001 | <.001 | <.001 | |
| Substantial arm swelling or tenderness, No/total No. (%) | |||||
| Sentinel lymph node biopsy | 15/412 (4) | 47/388 (12) | 22/389 (6) | 29/387 (7) | 20/381(5) |
| Standard axillary treatment | 17/387 (4) | 106/379 (28) | 55/379 (15) | 59/374 (16) | 42/362 (12) |
| NNT | 6.3 | 11.3 | 12.1 | 15.7 | |
| P‡ | <.001 | <.001 | <.001 | .002 | |
| Substantial numbness on ipsilateral side, No./total No. (%) | |||||
| Sentinel lymph node biopsy | 4/408 (1) | 36/388 (9) | 30/391 (8) | 35/389 (9) | 23/386 (6) |
| Standard axillary treatment | 10/385 (3) | 113/381 (30) | 101/380 (27) | 100/377 (27) | 74/363 (20) |
| NNT | 4.9 | 5.3 | 5.7 | 6.9 | |
| P‡ | <.001 | <.001 | <.001 | <.001 | |
| FACT-B+4 score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 127.5 (125.8 to 129.2) | 126.0 (123.9 to 128.1) | 128.0 (125.9 to 130.1) | 127.9 (125.7 to 130.0) | 132.7 (130.9 to 134.6) |
| Standard axillary treatment | 129.1 (127.4 to 130.8) | 121.7 (119.6 to 123.8) | 125.2 (123.0 to 127.3) | 125.4 (123.1 to 127.6) | 130.5 (128.4 to 132.6) |
| P† | <.001 | .001 | .003 | .002 | |
| STAI score, mean (95% CI) | |||||
| Sentinel lymph node biopsy | 43.4 (42.1 to 44.7) | 37.4 (36.1 to 38.7) | 35.9 (34.7 to 37.2) | 36.0 (34.7 to 37.2) | 33.7 (32.5 to 35.0) |
| Standard axillary treatment | 42.9 (41.5 to 44.2) | 37.4 (36.1 to 38.6) | 36.3 (35.1 to 37.5) | 36.1 (34.8 to 37.4) | 34.2 (32.9 to 35.4) |
| P§ | .77 | .37 | .61 | .35 |
Based on up to 424 patients allocated to sentinel lymph node biopsy and up to 405 patients allocated to standard management. CI = confidence interval; FACT-B+4 = Functional Assessment of Cancer Therapy-Breast +4 questionnaire; STAI = Spielberger State/Trait Anxiety Inventory; NNT = number needed to treat to obtain benefit.
Two-sided Student's t test comparing changes from baseline between groups.
Two-sided chi-square test.
Two-sided analysis of covariance.
The proportion of patients for whom the TOI score decreased from baseline by at least five points, a change that is considered to be clinically meaningful ( 25 ) , was statistically significantly higher in the standard treatment group than in the sentinel lymph node biopsy group at all time points ( P <.001, at 1 and 3 months after surgery; P = .002, 6 months after surgery; P = .001, 12 months after surgery).
Arm functioning subscale. Before surgery (i.e., at baseline), 78.1% of patients allocated to the standard treatment group and 77.5% of patients allocated to the sentinel lymph node biopsy group had the maximal arm functioning score of 20. Arm functioning scores from the mailed follow-up questionnaires were compared with baseline scores to examine change over time. Compared with baseline, arm functioning at 1, 3, 6, and 12 months after surgery was worse in both groups, but the impairment was greater in the standard group than in the sentinel lymph node biopsy group ( P <.001). For patients in both groups, arm functioning was lowest at 1 month after surgery. Mean arm functioning subscale scores at 1 month were 17.6 for the sentinel lymph node biopsy group and 14.6 for the standard axillary treatment group. Scores in both groups improved over time and by 12 months after surgery, the arm functioning scores of patients in the in the sentinel lymph node biopsy group were similar to those recorded at baseline ( Table 4 ).
Arm swelling and sensory loss on the ipsilateral side were considered a priori to be particularly important side effects of axillary surgery. Responses for these items on the arm functioning subscale categorized as “very much,” “quite a bit,” and “somewhat” were considered to be clinically significant whereas responses of “a little” or “none” were not. Proportions of patients in each group who reported substantially swollen or tender arms or substantial numbness are shown in Table 4 . After surgery, statistically significantly more patients in the standard treatment group than in the sentinel lymph node biopsy group reported swelling ( P <.001 for 1, 3, 6 months after surgery, P = .002 for 12 months after surgery) and numbness ( P <.001 for 1, 3, 6, 12 months after surgery).
FACT-B+4 score. Baseline FACT-B+4 scores were compared with scores at follow-up visits to examine change over time. The change in scores from baseline was statistically significantly less favorable for the standard treatment group than for the sentinel node biopsy group at 1 month ( P <.001), 3 months ( P = .001), 6 months ( P = .003), and 12 months ( P = .002) after surgery ( Table 4 ). For both groups, FACT-B+4 scores were lowest at 1 month after surgery and improved at follow-ups ( Table 4 ). The FACT-B+4 scores of patients in the standard treatment group took a longer time to return to the approximate baseline values compared with those of patients in the sentinel lymph node biopsy group (12 months versus 6 months).
STAI score. There was no difference between the treatment groups at any time point in the mean trait anxiety score or the mean state anxiety score ( Table 4 ).
Local Recurrences and Deaths
At 12 months after surgery, four patients in the standard treatment group and one patient in the sentinel node biopsy group had an axillary local recurrence (difference = 2.7%, 95% CI = −1.5% to 7.8%). The axillary recurrence in the sentinel lymph node biopsy group occurred in a sentinel lymph node–negative patient who initially received no further axillary therapy. The three axillary local recurrences in the standard treatment arm occurred in one patient who had a positive four-node sample with one involved node and who had axillary radiotherapy, and in two patients who underwent axillary clearance, one of whom was node negative and the other of whom had three positive nodes and refused radiation to the axilla.
There were seven deaths in the standard treatment group (two of which were due to metastatic breast carcinoma) and seven deaths in the sentinel node group (two of which were due to metastatic breast carcinoma).
D ISCUSSION
These results confirm previous preliminary observations from nonrandomized studies that suggested that sentinel lymph node biopsy is associated with less arm and shoulder morbidity than standard axillary treatment ( 10 – 14 ) . Evaluation and comparison of quality-of-life outcomes showed that sentinel lymph node biopsy was associated with better quality of life and reduced incidence of arm swelling and sensory morbidity compared with standard axillary treatment. These differences were evident in the intention-to-treat analysis, even though 17% of patients in the sentinel lymph node biopsy group underwent complete axillary clearance. Furthermore, because 25% of patients in the standard treatment group underwent the less extensive procedure of four-node sampling, the true morbidity of axillary clearance is underestimated by looking at the standard axillary treatment group as a whole. Thus, this trial probably underestimates the magnitude of the benefit of sentinel lymph node biopsy over axillary dissection. Our data confirm results from the randomized study reported by Veronesi et al. ( 17 ) , in which a difference in arm morbidity was found in favor of the sentinel lymph node biopsy group, albeit with the use of a simple questionnaire rather than with a validated quality-of-life instrument.
The other major morbidity of axillary surgery is lymphedema, a well-known complication of axillary dissection, with rates that range from 10% to 20% ( 33 ) . Our data at all time points showed that sentinel lymph node biopsy results in less patient-reported arm swelling than standard axillary treatment. Although the objective arm measurements were worse in the standard treatment group than in the sentinel lymph node biopsy group up to 6 months after surgery, they did not differ statistically significantly between the group at 12 months after surgery.
Patient-reported arm numbness or sensory deficit was common after standard axillary treatment but not after sentinel lymph node biopsy, even though the sentinel lymph node biopsy group included patients who underwent a secondary axillary surgery. Not surprisingly, the actual extent of sensory deficit among patients who reported a sensory loss was similar in the two groups. Clinicians' assessments of intercostobrachial nerve damage revealed that although both groups showed improvement in the severity of sensory deficit over time, the sentinel lymph node biopsy group had less sensory loss than the standard treatment group at all time points.
Our study corroborates previous findings from nonrandomized studies ( 12 – 16 ) that suggested that patients experience less impairment of shoulder movement after sentinel lymph node biopsy than after standard axillary surgery, and it showed that the standard treatment group had statistically significant impairment in shoulder flexion and abduction at 1 month after surgery compared with that in the sentinel lymph node biopsy group. However these impairments improved over time, possibly because of reduction in wound pain and discomfort, and possibly because of the beneficial effects of exercise and physiotherapy ( 33 ) . The early impairment of shoulder movements with rapid improvement was also seen in a smaller randomized study of sentinel lymph node biopsy versus axillary lymph node dissection ( 34 ) . There were no statistically significant differences between patients in the two groups in the range of motion for shoulder internal and external rotation.
A major potential benefit of reducing morbidity of axillary surgery is a reduction in health care costs. There was substantially less drain usage among patients in the sentinel lymph node biopsy group than among patients in the standard treatment group, an unsurprising finding given that drains are used only in completion axillary lymph node dissection patients. The advantages of eliminating wound drainage after axillary surgery include fewer clinic visits, increased patient satisfaction, and reduced costs. Cost containment is a major focus for health services. Sentinel lymph node biopsy patients spent less time in the hospital than standard axillary treatment patients. Inpatient stays in this study were longer than those in the United States but were typical for those in the British National Health Service ( 35 ) .
The morbidities we described above will have both societal and patient economic effect. The socioeconomic impact of axillary surgery reflects the complexity of the operation. Sentinel lymph node biopsy patients returned to normal domestic activity sooner than patients who had standard axillary surgery. This faster return to activity may be due partly to omitting axillary drainage, but is most likely due to the minimal invasive surgery with smaller wounds that produced less pain and discomfort. After surgery, most patients were offered adjuvant radiotherapy to the ipsilateral breast following wide local excision and/or treatment with systemic hormone and chemotherapy (which was based on nodal staging or tumor characteristics), thus further delaying the return of some patients in both groups to paid work. Our findings suggest that sentinel lymph node biopsy should reduce overall health care costs because the increased costs associated with sentinel lymph node biopsy, such as the capital equipment and consumable costs, should be quickly offset by savings associated with decreased hospital stays and faster returns to normal activity. A full economic analysis of this trial will be reported elsewhere.
Overall patient quality of life, as determined by the FACT-B+4 questionnaire responses, was found to be better at all time points after sentinel lymph node biopsy than after standard axillary treatment. TOI scores and arm functioning scores were lowest for both groups at 1 month after surgery and improved after that point, but throughout the follow-up period, the scores differed between the groups in favor of the sentinel lymph node biopsy group. It is important that, despite the uncertainty about the possible need for further surgery or the possibility of involved nodes being missed by sentinel lymph node biopsy, these clear quality-of-life benefits were not at the expense of substantially increased anxiety.
It is premature to make a definitive comment on local recurrence rate or survival in the two groups. However, the 12-month follow-up data suggested that the sentinel lymph node biopsy group had a low local recurrence rate and survival that was similar to that of the standard treatment group. A large nonrandomized study from Memorial Sloan Kettering, New York, reported only 0.1% local recurrence in sentinel lymph node–negative patients at a median follow-up of 31 months ( 37 ) , and an Italian study of 953 patients had a similar recurrence rate of 0.3% at a median follow-up of 38 months ( 36 ) .
Overall, our results are quantitatively similar to those found in a smaller single-institution randomized study from Milan ( 17 ) . There are, however, important differences between these two studies. The Milan trial excluded male breast cancer patients, patients with tumors larger than 2 cm, and patients who were scheduled to undergo mastectomy. Our trial included these three groups of patients ( 19 , 38 ) and is therefore more generalizable. In the Milan trial, sentinel lymph node biopsy was performed by using the radioisotope technique only, and immunohistochemical staining and serial sectioning were used intraoperatively to assess the sentinel lymph nodes and sentinel lymph node–positive patients underwent immediate clearance. By contrast, the approach in our study was to use the combination of sentinel node localization and routine histopathology (with hematoxylin–eosin), with delayed axillary node clearance for most sentinel node–positive patients. This management most closely resembles routine practice outside a large single center such as the European Institute of Oncology in Milan which uses pathology, a practice not used in community centers. Also, in the Milan study, arm morbidity was evaluated in a subset of 200 patients who underwent sentinel lymph node biopsy alone (i.e., sentinel lymph node–negative patients) and was not analyzed on an intention-to-treat basis. In our study, by contrast, patients were analyzed on intention-to-treat basis. In the Milan study, analyses were based on responses from interviews and a simple questionnaire, and no validated quality-of-life instrument was used. Our quality-of-life dataset of more than 800 patients is four times larger than the Milan dataset. This is also is the only multicenter randomized trial comparing sentinel lymph node biopsy with standard axillary treatment that has reported its findings. The unique features of this trial were the detailed standardized training program for all surgeons, which involved in-house training with the requirement to reach a preset standard for acceptance into the randomized phase ( 7 ) , and the direct comparison of sentinel lymph node biopsy with four-node sampling, which will be analyzed separately.
Our study has several limitations. First, the inclusion of four-node sampling techniques in the standard treatment arm may have led to an underestimate of the benefit of sentinel lymph node biopsy. Nonetheless, there was clear advantage in favor of sentinel lymph node biopsy for the quality-of-life and patient-reported variables. Second, the objective measurements showed smaller or no differences at 12 months, a result that seems discordant with patient-reported quality of life. We anticipated that measurements such as arm volume or shoulder angles would provide a more objective measure of the benefits of sentinel lymph node biopsy than patient- or clinician-assessed outcomes, including those based on quality-of-life scales (albeit validated ones), given that blind assessment by the clinician was problematic and by the patient, impossible. However, the objective measurements proved to be less informative than we had hoped, mainly because of the high residual variances throughout the analyses, which strongly suggest a great degree of measurement variation. Conversely, clinician- and patient-assessed outcomes, including those based on quality-of-life scales, showed a large, and largely persistent, advantage to sentinel lymph node biopsy, that could not be plausibly attributed to nonblind assessment alone. We checked for between-center variability but found no evidence of any statistically significant heterogeneity.
In conclusion, this study has shown that sentinel lymph node biopsy is a safe and effective alternative to routine axillary dissection for nodal staging in early-stage breast cancer. Compared with standard axillary treatment, sentinel lymph node biopsy was associated with reduced arm morbidity and better quality of life with no increase in anxiety. These conclusions have been endorsed in a report by an expert panel under the auspices of the American Society of Clinical Oncology ( 39 ) . Our results support the introduction of this minimally invasive staging procedure in operable breast cancer. In the United Kingdom, a national sentinel lymph node biopsy training program (NEW START) is in progress ( http://www.rcseng.ac.uk/education/courses/new_start.html ).
A PPENDIX
Arm functioning subscale in FACT-B+4 patient self-assessment questionnaire
| Item . | Not at all . | A little . | Somewhat . | Quite a bit . | Very much . |
|---|---|---|---|---|---|
| One or both arms are swollen or tender | 0 | 1 | 2 | 3 | 4 |
| Movement of my arm on the operated side is painful | 0 | 1 | 2 | 3 | 4 |
| I have a poor range of arm movements on this side | 0 | 1 | 2 | 3 | 4 |
| My arm on this side feels numb | 0 | 1 | 2 | 3 | 4 |
| I have stiffness of my arm on this side | 0 | 1 | 2 | 3 | 4 |
| Item . | Not at all . | A little . | Somewhat . | Quite a bit . | Very much . |
|---|---|---|---|---|---|
| One or both arms are swollen or tender | 0 | 1 | 2 | 3 | 4 |
| Movement of my arm on the operated side is painful | 0 | 1 | 2 | 3 | 4 |
| I have a poor range of arm movements on this side | 0 | 1 | 2 | 3 | 4 |
| My arm on this side feels numb | 0 | 1 | 2 | 3 | 4 |
| I have stiffness of my arm on this side | 0 | 1 | 2 | 3 | 4 |
Arm functioning subscale in FACT-B+4 patient self-assessment questionnaire
| Item . | Not at all . | A little . | Somewhat . | Quite a bit . | Very much . |
|---|---|---|---|---|---|
| One or both arms are swollen or tender | 0 | 1 | 2 | 3 | 4 |
| Movement of my arm on the operated side is painful | 0 | 1 | 2 | 3 | 4 |
| I have a poor range of arm movements on this side | 0 | 1 | 2 | 3 | 4 |
| My arm on this side feels numb | 0 | 1 | 2 | 3 | 4 |
| I have stiffness of my arm on this side | 0 | 1 | 2 | 3 | 4 |
| Item . | Not at all . | A little . | Somewhat . | Quite a bit . | Very much . |
|---|---|---|---|---|---|
| One or both arms are swollen or tender | 0 | 1 | 2 | 3 | 4 |
| Movement of my arm on the operated side is painful | 0 | 1 | 2 | 3 | 4 |
| I have a poor range of arm movements on this side | 0 | 1 | 2 | 3 | 4 |
| My arm on this side feels numb | 0 | 1 | 2 | 3 | 4 |
| I have stiffness of my arm on this side | 0 | 1 | 2 | 3 | 4 |
We thank all patients in the ALMANAC study; research fellows; other study investigators; and all the surgery, nuclear medicine, radiological, radiographic, and nursing staff at each center.
R. E. Mansel was the principal investigator (PI), obtained funding for the trial, designed the study, and contributed to the interpretation of the data. L. Fallowfield was joint PI, contributed to protocol development, data collection and analysis for the quality of life assessments. M. Kissin recruited the largest number of patients into the study. A. Goyal wrote the first version of the manuscript and contributed to data collection, analysis and interpretation, and drafting and revision of the manuscript. R. G. Newcombe contributed to study design, did the statistical analyses, and reviewed the manuscript. M. Kissin, J. M. Dixon, C. Yiangou, K. Horgan, N. Bundred, I. Monypenny, D. England, M. Sibbering, T. I. Abdullah, L. Barr, U. Chetty, and D. H. Sinnett were center PIs, implemented the study at the local regional study sites, contributed to data collection, and offered critical revisions of the manuscript for important intellectual content. A. Fleissig contributed to analysis, interpretation and reporting of the quality-of-life data. D. Clarke contributed to study design, protocol, and reviewed the manuscript. P. Ell contributed to the optimization of radioisotope injection and lymphoscintigraphy technique and reviewed the manuscript.
The validation phase of the study was supported by a grant from the UK Medical Research Council. The randomized phase was supported by grants from the Welsh Office of Research and Development; National Cancer Research Network; GE Healthcare, Little Chalfont, England; and Wales Cancer Trials Network, and Cancer Research UK funds (L. Fallowfield). The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The steering committee had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Steering committee: Robert Haward (Chairman; Cookridge Hospital, Leeds); David Cohen (University of Glamorgan, Treforest); Ian Ellis (Nottingham City Hospital, Nottingham); Emiel Rutgers (The Netherlands Cancer Institute, Amsterdam); Robert Mansel-Principal Investigator (Cardiff University, Cardiff); Lesley Fallowfield (Cancer Research UK, Brighton); Ciaran Woodman (Christie Hospital, Manchester); Robert Newcombe (Cardiff University, Cardiff); Peter J. Ell (The Middlesex Hospital, London).
Data monitoring committee: Mahesh Parmar (Chairman; Medical Research Council, London); John Northover (St. Mark's Hospital, London); John Yarnold (The Royal Marsden, London).
Trial coordinator: Julia Townson (Cardiff University, Cardiff).
Quality-of-Life assessment (Cancer Research UK Psychosocial Oncology Group, Brighton): Data coordinator—Leigh Johnson; Data Manager—Carolyn Langridge; Research Fellows—Valerie Jenkins.
Participating centers, institution (investigators): Bangor, Gwynedd Hospitals NHS Trust (Derek Crawford); Birmingham, Queen Elizabeth Medical Centre (David England); Cardiff, University Hospital of Wales (Robert Mansel, Ian Monypenny, Helen Sweetland, David Webster); Derby, Derby City General Hospital (Mark Sibbering, Howard Holliday); Edinburgh, Western General Hospital (Utheshtra Chetty, J. Michael Dixon); Guildford, Royal Surrey County Hospital (Mark Kissin); Huddersfield, The Royal Infirmary (Richard Sainsbury); Hull, Castle Hill Hospital (Tapan Mahapatra); Leeds, Leeds General Infirmary (Kieran Horgan); London, Charing Cross Hospital (Dudley H. Sinnett); Manchester, South Manchester University Hospital (Lester Barr, Nigel Bundred); Nottingham, Nottingham City Hospital (James Geraghty); Portsmouth, Queen Alexandra Hospital (Constantinos Yiangou); Rhyl, Glan Clwyd Hospital (Christopher Davies); Swansea, Morriston Hospital (Mike Chare); Peterborough, Edith Cavell Hospital (Tholkifl Abdullah).
Funding to pay the Open Access publication charges for this article was provided by Cardiff University, Wales.
References
Maunsell E, Brisson J, Deschenes L. Arm problems and psychological distress after surgery for breast cancer.
Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer.
Roses DF, Brooks AD, Harris MN, Shapiro RL, Mitnick J. Complications of level I and II axillary dissection in the treatment of carcinoma of the breast.
Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema.
Warmuth MA, Bowen G, Prosnitz LR, Chu L, Broadwater G, Peterson B, et al. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey.
The UK NHS Breast Screening Programme
Clarke D, Newcombe RG, Mansel RE. The learning curve in sentinel node biopsy: the ALMANAC experience.
McMasters KM, Wong SL, Chao C, Woo C, Tuttle TM, Noyes RD, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques.
Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma.
Temple LK, Baron R, Cody HS, III, Fey JV, Thaler HT, Borgen PI, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women.
Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer.
Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma.
Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer.
Haid A, Koberle-Wuhrer R, Knauer M, Burtscher J, Fritzsche H, Peschina W, et al. Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: a comparative evaluation.
Peintinger F, Reitsamer R, Stranzl H, Ralph G. Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients.
Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer.
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer.
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer.
Mansel RE, Goyal A. European studies on breast lymphatic mapping.
Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer—results of the ALMANAC validation phase. Breast Cancer Res Treat. Epub March
Steele RJ, Forrest AP, Gibson T, Stewart HJ, Chetty U. The efficacy of lower axillary sampling in obtaining lymph node status in breast cancer: a controlled randomized trial.
Kuhnke E. Determination of volume by measuring the circumferences.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument.
Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively.
Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592.
Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale.
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial.
Spielberger C. The manual for the state-trait inventory. Palo Alto (CA): Consulting Psychologists Press;
Wilson EB. Probable inference, the law of succession, and statistical inference.
Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods.
Goyal A, Newcombe RG, Mansel RE. Clinical relevance of internal mammary node drainage in sentinel node biopsy for breast cancer.
Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients.
Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial.
Gemignani ML, Cody HS, III, Fey JV, Tran KN, Venkatraman E, Borgen PI. Impact of sentinel lymph node mapping on relative charges in patients with early-stage breast cancer.
Veronesi U, Galimberti V, Mariani L, Gatti G, Paganelli G, Viale G, et al. Sentinel node biopsy in breast cancer: early results in 953 patients with negative sentinel node biopsy and no axillary dissection.
Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures.
Goyal A, Horgan K, Kissin M, Yiangou C, Sibbering M, Lansdown M, et al. Sentinel lymph node biopsy in male breast cancer patients.