Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.303
Revised: October 7, 2013
Accepted: November 1, 2013
Published online: January 7, 2014
AIM: To evaluate the effect of low central venous pressure (LCVP) on blood loss and blood transfusion in patients undergoing hepatectomy.
METHODS: Electronic databases and bibliography lists were searched for potential articles. A meta-analysis of all randomized controlled trials (RCTs) investigating LCVP in hepatectomy was performed. The following three outcomes were analyzed: blood loss, blood transfusion and duration of operation.
RESULTS: Five RCTs including 283 patients were assessed. Meta-analysis showed that blood loss in the LCVP group was significantly less than that in the control group (MD = -391.95, 95%CI: -559.35--224.56, P < 0.00001). In addition, blood transfusion in the LCVP group was also significantly less than that in the control group (MD = -246.87, 95%CI: -427.06--66.69, P = 0.007). The duration of operation in the LCVP group was significantly shorter than that in the control group (MD = -18.89, 95%CI: -35.18--2.59, P = 0.02). Most studies found no significant difference in renal and liver function between the two groups.
CONCLUSION: Controlled LCVP is a simple and effective technique to reduce blood loss and blood transfusion during liver resection, and appears to have no detrimental effects on liver and renal function.
Core tip: The morbidity and mortality after hepatic resection have been reported to correlate with excessive intraoperative blood loss and blood transfusion. This meta-analysis showed that controlled low central venous pressure is a simple and effective technique to reduce blood loss and blood transfusion during liver resection, and appears to have no detrimental effects on liver and renal function.
- Citation: Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol 2014; 20(1): 303-309
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.303
Hepatectomy is widely used in patients with primary liver cancer, secondary liver cancer, hepatic hemangioma, and other liver diseases[1-5]. The most common and crucial complication of hepatectomy is hemorrhage[6,7]. The morbidity and mortality after hepatic resection have been reported to correlate with excessive intraoperative blood loss and blood transfusion[8-10]. Because in most cases the Pringle maneuver is employed to control blood inflow to the liver, blood loss during liver resection is mainly from hepatic veins. The blood loss volume during hepatic resection is related to central venous pressure (CVP)[11,12]. Although there is some evidence that controlled low central venous pressure (LCVP) can decrease blood loss in patients undergoing liver resection, this technique remains controversial due to its potential risks. This meta-analysis aimed to evaluate the role of controlled LCVP during liver resection.
Computerized searches of PubMed, EMBASE, the Cochrane Library and Google Scholar were conducted up to November 2012. The search words used were “central venous pressure” and “liver resection” or “hepatic dissection” or “hepatectomy”. We also performed a full manual search of the bibliographies of each peer-reviewed paper selected. No language or date limitations were imposed. Furthermore, there was no limitation on publication form.
All the articles were reviewed independently by two authors (Li Z and Sun YM). Any disagreement regarding whether a study should be selected was resolved by consensus. Inclusion criteria were established at the beginning of the study and were as follows: (1) Population: humans undergoing hepatic dissections; (2) Intervention: LCVP by any method; (3) Outcome: outcomes included intraoperative blood loss; and (4) Methodology: randomized controlled trials only.
The authors (Li Z and Sun YM) analyzed the quality of methodology in each study independently. The methods of randomization and concealment of allocation were assessed for each study. The level of blinding was evaluated for each trial. A study was recognized as double-blind if it stated that both the patients and study participants did not recognize which group the patients were allocated to. Completeness of follow-up was evaluated for each study. The status at follow-up was described as the proportion of subjects who had clinical outcomes. Is this correct? A methodology score was given to each study using a modified method reported by Jadad et al[13]. One point was added to a study if there was a clear description of “withdrawals and dropouts”. For the studies with complete follow-up, one point was given if there was an appropriate description of withdrawals and dropouts[14].
Two authors (Li Z and Sun YM) performed data extraction for each study independently. Any disagreement on data extraction was resolved by consensus. Detailed information on population, intervention, and outcomes were recorded, including sample size, surgical procedure, methods of controlled LCVP, transfusion trigger, blood loss, volume of transfusion, and complications.
The primary outcome analyzed was intraoperative blood loss. Secondary outcomes included volume of intraoperative blood transfusion, and duration of operation. Statistical analysis was carried out using the software RevMan 5.2 (Cochrane Collaboration, Oxford, England). Outcomes were measured in a standard way as mean±standard deviation across the studies. A random effect model was used if there was significant heterogeneity (P < 0.05) between results across studies. A fixed effect model was used if there was no significant heterogeneity (P > 0.05). Heterogeneity was assessed using the Cochrane χ2 text (using a 10% significance level) and the I2 statistic (percentage of variation due to heterogeneity with higher values indicating a greater degree of heterogeneity). The OR for the results was presented with 95%CI.
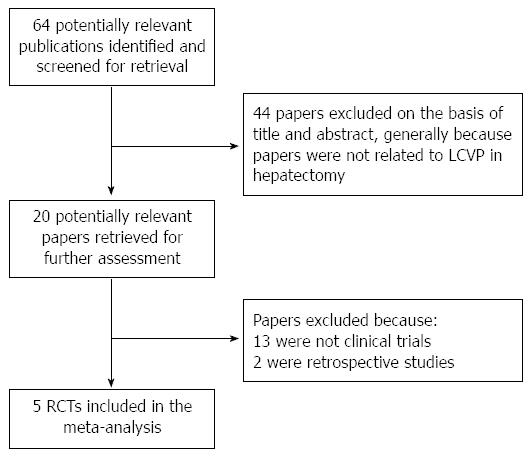
We identified 64 studies through an electronic search. Five studies fulfilled the inclusion criteria of the meta-analysis (Figure 1)[15-19]. The characteristics of the 5 selected studies are shown in Table 1. The 5 studies involved 283 subjects, including 141 patients who underwent LCVP, and 142 who served as controls. The methods of controlled LCVP included Trendelenburg’s posture, nitroglycerine, furosemide, fentanyl, control of infusion speed, and clamping the infrahepatic vena cava (IVC). The transfusion trigger was Hb < 80 g/L in two studies and in another study the transfusion trigger was set as blood loss exceeding 25% of the blood volume or Hb < 80 g/L. The Jadad scores ranged from 1 to 4.
| Ref. | Disease | Sample size(LCVP/control) | LCVP technique | Transfusion trigger | Methodology score | Blinding (patient/surgeon/anesthesiologist) | Lost to follow-up |
| Liu et al[15] 2008 | Hepatocellular carcinoma | 23/23 | Trendelenburg’s posture, nitroglycerine, furosemide, control of infusion speed | Hb < 80 g/L | 3 | Yes/no/no | 0/46 |
| Wang et al[16] 2006 | Hepatocellular carcinoma | 25/25 | Trendelenburg’s posture, limiting the volume of infusion, nitroglycerine, furosemide | Hb < 80 g/L | 4 | Yes/no/no | 0/52 |
| Kato et al[17] 2008 | Primary liver cancer, metastatic liver tumor | 43/42 | Clamping the infrahepatic inferior vena cava | Not reported | 2 | No/no/no | 0/85 |
| Liu et al[18] 2005 | Not reported | 30/30 | Trendelenburg’s posture, isoflurane, fentanyl, limiting the volume of infusion, nitroglycerine | Blood loss exceeding 25% of the blood volume or Hb < 80 g/L | 1 | No/no/no | 0/50 |
| El-Kharboutly et al[19] 2004 | Not reported | 20/20 | Nitroglycerine | Not reported | 3 | No/no/no | 0/40 |
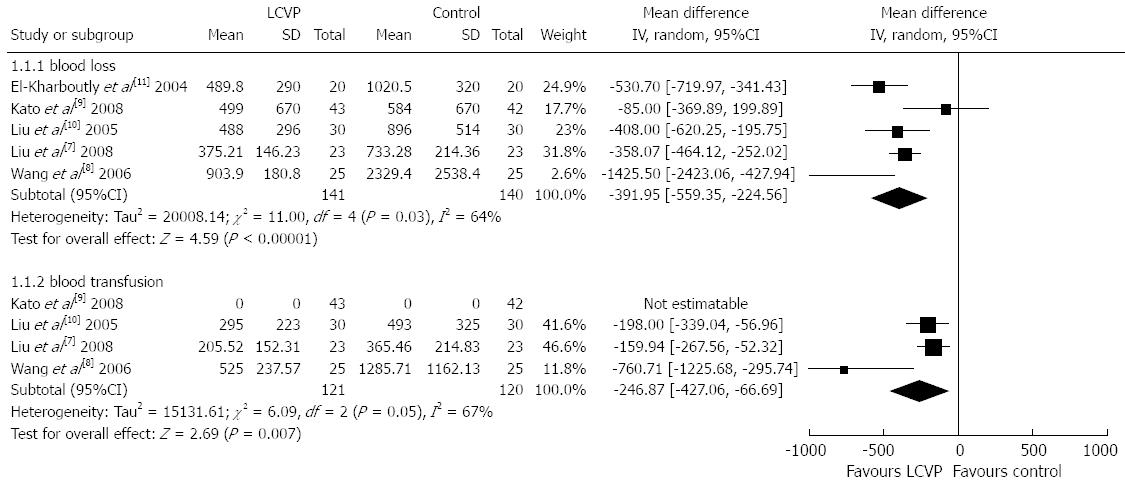
We first analyzed blood loss in the two groups. All five studies reported blood loss. There was statistical heterogeneity among the five trials and the random-effect model was used (χ2 = 11.0, P = 0.03, I2 = 64%). Meta-analysis showed that blood loss in the LCVP group was significantly less than that in the control group (MD = -391.95, 95%CI: -559.35--224.56, P < 0.00001), (Figure 2).
Secondly, we analyzed blood transfusion in the two groups. Four studies reported blood transfusion. There was statistical heterogeneity among the four trials and the random-effect model was used (χ2 = 6.09, P = 0.05, I2 = 67%). Meta-analysis showed that blood transfusion in the LCVP group was significantly less than that in the control group (MD = -246.87, 95%CI: -427.06--66.69, P = 0.007) (Figure 2).
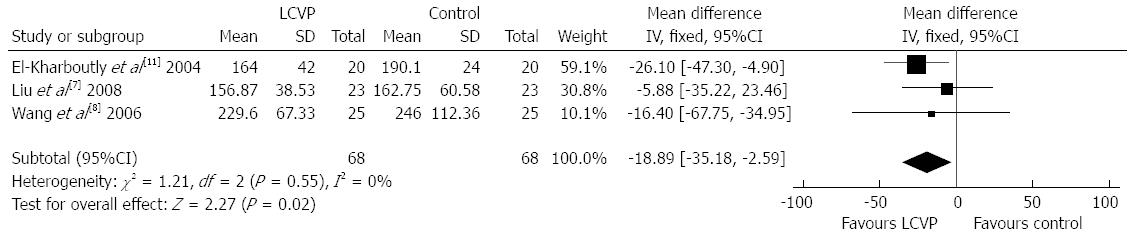
The duration of operation was also evaluated. Three trials reported duration of operation. There was no statistical heterogeneity among the three trials and the fixed-effect model was used (χ2 = 1.21, P = 0.55, I2 = 0%). Meta-analysis revealed that the duration of operation in the LCVP group was significantly shorter than that in the control group (MD = -18.89, 95%CI: -35.18--2.59, P = 0.02) (Figure 3).
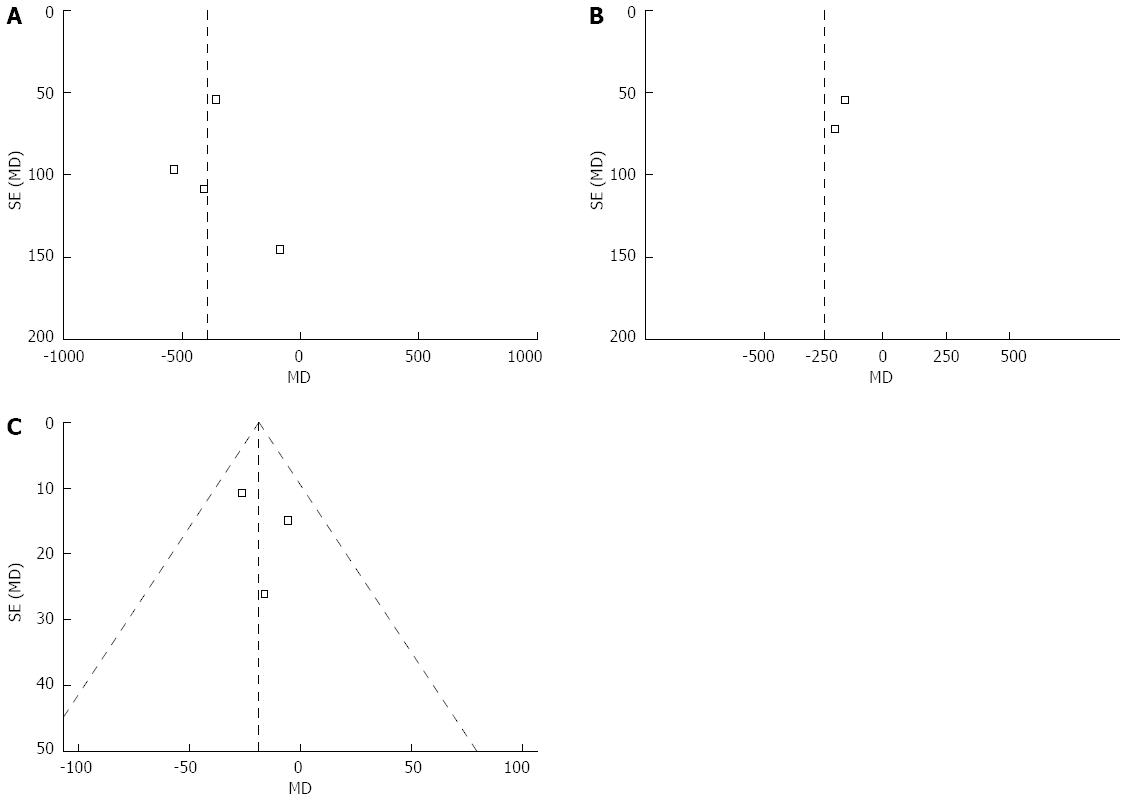
The funnel plots for blood loss, blood transfusion, and duration of operation showed asymmetry, suggesting the possibility of publication bias (Figure 4).
A sensitivity analysis was performed and included only the studies published as full texts, which excluded one study[19]. Meta-analysis showed that blood loss and blood transfusion in the LCVP group were both significantly less than those in the control group (Table 2). Meta-analysis also revealed that there was no difference regarding duration of operation between the two groups (Table 2).
| Studies (n) | MD (95%CI) | P value | |
| Studies published in full texts | 4 | ||
| Blood loss (mL) | 4 | -348.24 (-553.34, -143.14) | 0.0009 |
| Blood transfusion (mL) | 4 | -246.87 (-427.06, -66.69) | 0.007 |
| Duration of operation (min) | 2 | -8.47 (-33.94, 17.01) | 0.51 |
| Studies published in English | 4 | ||
| Blood loss (mL) | 4 | -394.84 (-624.14, -165.53) | 0.0007 |
| Blood transfusion (mL) | 3 | -416.00 (-998.29, 166.30) | 0.16 |
| Duration of operation (min) | 3 | -18.89 (-35.18, -2.59) | 0.02 |
| High-quality studies | 3 | ||
| Blood loss (mL) | 3 | -488.64 (-730.38, -246.89) | < 0.0001 |
| Blood transfusion (mL) | 2 | -416.00 (998.29, 166.30) | 0.16 |
| Duration of operation (min) | 3 | -18.89 (-35.18, -2.59) | 0.02 |
Secondly, only studies published in English were included. Meta-analysis showed that blood loss and duration of operation in the LCVP group were both significantly less than those in the control group (Table 2). Meta-analysis also revealed that there was no difference regarding blood transfusion between the two groups (Table 2).
Finally, only three high-quality studies[15,18,19] were analyzed. Meta-analysis showed that blood loss and duration of operation in the LCVP group were both significantly less than those in the control group (Table 2). Meta-analysis also revealed that there was no difference regarding blood transfusion between the two groups (Table 2).
Patient harm measures are summarized in Table 3. The recorded harm parameters included renal and liver function, postoperative morbidity and mortality, and hemodynamic stability. All the studies reported no significant difference in renal and liver function between the LCVP group and the control group, except one study which observed that blood urea nitrogen (BUN) was significantly higher in the control group than in the LCVP group, but was within the normal range in both groups. In two studies, there was no significant difference in morbidity and mortality between the two groups. One study found that systolic blood pressures in the LCVP group were lower than those in the control group[18].
| Ref. | Harm measure | Results |
| Liu et al[15] 2008 | Renal function | No significant differences in BUN and Cr on pre- and post-operative d 1, 3 and 7 between LCVP and control group. Values all within the normal range in both groups |
| Wang et al[16] 2006 | Liver and renal function | There were no significant differences in ALT, TBIL, and Cr on post-operative d 1, 3, and 7 between the two groups. BUN was significantly higher in the control group than in the LCVP group, but was within the normal range in both groups |
| Post-operative morbidity | Post-operative complications included biliary fistula, gastrointestinal bleeding, pleural effusion and subphrenic fluid collection, with an incidence of 20% (5/25) in the LCVP group and 24% (6/25) in the control group | |
| Kato et al[17] 2008 | Renal function | There were no significant intergroup differences in the values of BUN and creatinine on postoperative 1, 3, and 5 d |
| Postoperative morbidity and mortality | There was no morbidity related to IVC clamping and no mortality in the two groups | |
| Liu et al[18] 2005 | Renal functionHemodynamic stability | No significant differences in BUN and Cr at postoperative 24 h between the two groups. Systolic blood pressures in the LCVP group were lower than those in the control group |
| El-Kharboutly et al[19] 2004 | Not reported | Not reported |
Hepatectomy is a major abdominal surgical procedure with a risk of significant blood loss and subsequent blood transfusion, which are strongly correlated with postoperative morbidity and mortality[20]. In addition, intra- or post-operative massive blood loss and transfusion carry potential risks of infectious disease, acute respiratory distress syndrome, coagulation disorder, multiple organ failure, and may also promote tumor recurrence due to its inhibitory effect on immunity[21]. Therefore, various methods, such as Pringle’s maneuver, normothermic total hepatic vascular exclusion and unilateral hepatic hilum occlusion, have been used to reduce intra-operative hemorrhage. Recently, it was reported that intraoperative blood loss volume is correlated with CVP[11,12]. LCVP in liver resection is generally referred to as CVP lower than 5 mmHg. Some retrospective studies reported that LCVP during hepatectomy can reduce intraoperative blood loss and decrease the rate of postoperative complications[11,22].
In the present meta-analysis, by maintaining CVP under 5 mmHg during liver resection with Trendelenburg’s posture, administration of drugs and control of infusion speed, and clamping the infrahepatic IVC, blood loss and transfusion requirements were significantly reduced in the LCVP group compared with the control group.
We found that in one study[16], patients in the LCVP and control group had a blood loss of 903.9 mL and 2329.4 mL, respectively; and blood transfusion of 525 mL and 1285.71 mL in the LCVP and control group, respectively. However, in another study[17], both groups did not require blood transfusion. Hence, blood loss and blood transfusion varied widely in different studies. In addition, the study with the greatest blood loss and blood transfusion also had longer duration of operation than the other studies. A potential reason for this may be that the surgeons in this study had less surgical experience than those in other studies. However, this study also showed that blood loss and blood transfusion in the LCVP group were both significantly less than those in the control group.
Furthermore, this meta-analysis revealed that the duration of operation in the LCVP group was significantly less than that in the control group. It is understandable that less blood loss is favorable for the surgical visual field and procedure, and reduces the duration of operation. Nevertheless, complicated or difficult operations usually take longer and result in more blood loss and blood transfusion. Thus, LCVP may be necessary for complicated or difficult operations. For uncomplicated operations, especially when the operators are experienced surgeons, despite the fact that LCVP can reduce blood loss and blood transfusion, the duration of operation may be short and LCVP might not be necessary.
Four studies recorded harm measures, and all found no difference between the LCVP group and the control group, with the exception of one study which found that BUN was significantly higher in the control group than in the LCVP group, but within the normal range[16]. However, during LCVP, mean blood pressure can decrease to less than 65 mmHg, which will reduce blood inflow to the organs, and may lead to organ ischemic and reperfusion injury. The routine measurement of renal and liver function is not sensitive enough to detect mild ischemic injury and reperfusion injury. Therefore, further research may be required in the future.
However, several recent studies reported that CVP did not correlate with blood loss during living donor hepatectomy[23-26]. For example, CVP during hepatic resection was not associated with intraoperative blood loss in living liver donors[25,26], and intraoperative hemorrhage was not reduced significantly in patients with relatively low CVP[23,24]. Discrepancies among these studies may arise from differences in patient populations. The hemorrhagic tendency of liver tissue in living donors may be different from that in patients with benign or malignant hepatic lesions.
There were several limitations in the present meta-analysis. First, the number of trials was inadequate. Second, there was heterogeneity among trials; there were a number of factors causing heterogeneity, such as different methods of LCVP. Third, some individual trials were of low quality. Therefore, further large RCTs are needed.
In conclusion, controlled LCVP is a simple and effective technique to reduce blood loss and blood transfusion during liver resection, and appears to have no detrimental effects on liver and renal function.
The most common and crucial complication of hepatectomy is hemorrhage. The morbidity and mortality after hepatic resection have been reported to correlate with excessive intraoperative blood loss and blood transfusion.
Although there is some evidence that controlled low central venous pressure (LCVP) can reduce blood loss in patients undergoing liver resection, this technique remains controversial due to its potential risks.
The authors performed a meta-analysis of randomized controlled trials to evaluate the role of controlled LCVP during liver resection.
Controlled LCVP is a simple and effective technique to reduce blood loss and blood transfusion during liver resection. Hepatectomy should be conducted under controlled LCVP.
This study evaluated the effect of LVCP on blood loss and blood transfusion in patients undergoing hepatectomy by conducting a meta-analysis. The findings are significant in studies of liver neoplasm.
P- Reviewers: Cheng JK, Yu HP S- Editor: Qi Y L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Hart ME, Precht A. Robotic liver resection technique. Cancer J. 2013;19:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | DeOliveira ML, Kambakamba P, Clavien PA. Advances in liver surgery for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Pathak S, Dash I, Taylor MR, Poston GJ. The surgical management of neuroendocrine tumour hepatic metastases. Eur J Surg Oncol. 2013;39:224-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Tzeng CW, Aloia TA. Colorectal liver metastases. J Gastrointest Surg. 2013;17:195-201; quiz p.201-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Slakey DP, Simms E, Drew B, Yazdi F, Roberts B. Complications of liver resection: laparoscopic versus open procedures. JSLS. 2013;17:46-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kim SH, Kim YK. Improving outcomes of living-donor right hepatectomy. Br J Surg. 2013;100:528-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Otsubo T. Control of the inflow and outflow system during liver resection. J Hepatobiliary Pancreat Sci. 2012;19:15-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 227] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Yanaga K, Kanematsu T, Takenaka K, Matsumata T, Yoshida Y, Sugimachi K. Hepatic resection for hepatocellular carcinoma in elderly patients. Am J Surg. 1988;155:238-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998;85:188-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 287] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12275] [Cited by in F6Publishing: 12441] [Article Influence: 444.3] [Reference Citation Analysis (0)] |
| 14. | Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1792] [Cited by in F6Publishing: 1661] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Cai M, Duan S, Peng X, Lai Y, Li Y. Effect of controlled low central venous pressure on renal function in major liver resection. Chin-Germ J Clin Oncol. 2008;7:7-9. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 16. | Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935-939. [PubMed] [Cited in This Article: ] |
| 17. | Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T. Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World J Surg. 2008;32:1082-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Liu HZ, Zhou QL, Wang XH, Yang CX, Xu YH. Application of low central venous pressure in liver resection. Zhonghua Gandan Waike Zazhi. 2005;11:461-463. [Cited in This Article: ] |
| 19. | El-Kharboutly WS, El-Wahab MA. The role of adoption of low central venous pressure in hepatic resection with pringle manoeuvre in reducing blood loss and improving operative outcome. Egyp J Anaesth. 2004;20:369-376. [Cited in This Article: ] |
| 20. | Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, Frena A, Morganti M, Ercolani G, Masetti M. Liver resection without blood transfusion. Br J Surg. 1995;82:1105-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Mizuno S, Makuuchi M. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303-309. [PubMed] [Cited in This Article: ] |
| 22. | Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Niemann CU, Feiner J, Behrends M, Eilers H, Ascher NL, Roberts JP. Central venous pressure monitoring during living right donor hepatectomy. Liver Transpl. 2007;13:266-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Chhibber A, Dziak J, Kolano J, Norton JR, Lustik S. Anesthesia care for adult live donor hepatectomy: our experiences with 100 cases. Liver Transpl. 2007;13:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lutz JT, Valentín-Gamazo C, Görlinger K, Malagó M, Peters J. Blood-transfusion requirements and blood salvage in donors undergoing right hepatectomy for living related liver transplantation. Anesth Analg. 2003;96:351-35, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 26. | Kim YK, Chin JH, Kang SJ, Jun IG, Song JG, Jeong SM, Park JY, Hwang GS. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand. 2009;53:601-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |