Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2044
Peer-review started: December 28, 2016
First decision: February 10, 2017
Revised: February 15, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 21, 2017
To evaluate the correlation between subjective assessments of pancreatic hardness based on the palpation, objective measurements using a durometer, and magnetic resonance imaging (MRI) findings for assessing pancreatic hardness.
Eighty-three patients undergoing pancreatectomies were enrolled. An experienced surgeon subjectively evaluated the pancreatic hardness in the surgical field by palpation. The pancreatic hardness was also objectively evaluated using a durometer. Preoperative MRI findings were evaluated by a radiologist in terms of the apparent diffusion coefficient (ADC) values, the relative signal intensity decrease (RSID) of the pancreatic parenchyma, and the diameter of the pancreatic parenchyma and duct. Durometer measurement results, ADC values, RSID, pancreatic duct and parenchyma diameters, and the ratio of the diameters of the duct and parenchyma were compared between pancreases judged to be soft or hard pancreas on the palpation. A correlation analysis was also performed between the durometer and MRI measurements.
The palpation assessment classified 44 patients as having a soft pancreas and 39 patients as having a hard pancreas. ADC values were significantly lower in the hard pancreas group. The ductal diameter and duct-to-pancreas ratio were significantly higher in the hard pancreas group. For durometer measurements, a correlation analysis showed a positive correlation with the ductal diameter and the duct-to-pancreas ratio and a negative correlation with ADC values.
Hard pancreases showed lower ADC values, a wider pancreatic duct diameter and a higher duct-to-pancreas ratio than soft pancreases. Additionally, the ADC values, diameter of the pancreatic duct and duct-to-pancreas ratio were closely correlated with the durometer results.
Core tip: The texture of the pancreas is an important predictive factor for the development of postoperative complications following pancreatic surgery. Durometric measurements correlated well with surgeons’ palpation-based assessment of the hardness of pancreas and histologic evaluation for fibrosis and fat content. If we could estimate the texture of the pancreas preoperatively, it would be very helpful for surgeons to prepare for the possibility of postoperative pancreatic leakage or fistula. Preoperative magnetic resonance imaging (MRI) can be used to predict the hardness of pancreas. Apparent diffusion coefficient (ADC) values measured in MRI were significantly lower in the hard pancreas than soft pancreas. The ductal diameter and duct-to-pancreas ratio were significantly higher in the hard pancreas. For durometer measurements, a correlation analysis showed a positive correlation with the ductal diameter and the duct-to-pancreas ratio and a negative correlation with ADC values.
- Citation: Hong TH, Choi JI, Park MY, Rha SE, Lee YJ, You YK, Choi MH. Pancreatic hardness: Correlation of surgeon’s palpation, durometer measurement and preoperative magnetic resonance imaging features. World J Gastroenterol 2017; 23(11): 2044-2051
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2044
The texture of the pancreas is an important predictive factor for the development of postoperative complications following pancreatic surgery. Postoperative pancreatic leakage or fistula formation is more frequent in soft pancreases than hard pancreases[1-5]. The pancreatic texture is determined by a combination of fatty infiltration and fibrotic change. A decreased pancreatic fat content and increased fibrosis are related to a harder pancreatic texture. A hard pancreas resulting from fibrosis has a high suture-hold capacity and low pancreatic juice secretion; thus, pancreatic leaks or pancreatic fistulae occur less frequently in patients with a hard pancreas[3,4,6-8].
The texture of the pancreas can be evaluated based on the histologic characteristics of the pancreas, including the degrees of fibrosis and fatty infiltration. However, a considerable amount of time is necessary to acquire histologic results. Therefore, the most commonly used method to assess pancreatic hardness in practice is the surgeon’s subjective determination of pancreatic hardness during the operation. Given that a surgeon’s palpation-based determination may not be reproducible, objective measurement using a durometer was suggested[9]. Durometric measurements correlated well with surgeons’ palpation-based assessment and histologic evaluation for fibrosis and fat content[6,9]. One additional benefit of the durometer is that the durometer is easy to use, and quantitative assessment is possible.
The surgeon’s palpation-based determination and durometer measurement can both be performed intraoperatively. If we could estimate the texture of the pancreas preoperatively, it would be very helpful for surgeons to prepare for the possibility of postoperative pancreatic leakage or fistula. Magnetic resonance imaging (MRI) is one possible candidate for the preoperative evaluation of the texture of the pancreas. Diffusion-weighted imaging (DWI) can be applied to the pancreas to measure pancreatic fibrosis[10-12]. The degree of fibrosis is negatively correlated with apparent diffusion coefficient (ADC) values[10,11]. An increase in the fat content is noted in soft pancreases, and the fat content of the pancreas can be estimated using in- and opposed-phase MRI[13]. As pancreatic hardness is caused by increased fibrosis, parenchymal atrophy and duct dilatation resulting from fibrosis are also important parameters to predict pancreatic hardness[14]. Therefore, the aim of this study was to evaluate the correlation among the subjective palpation-based assessment, objective measurements using a durometer and MRI parameters for assessing pancreatic hardness.
This retrospective study was approved by the Institutional Review Board, and the requirement for informed consent was waived. From September 2014 to November 2016, 145 patients underwent some resection of the pancreas, including Whipple’s operation, pylorus-preserving pancreatoduodenectomy (PPPD), and distal pancreatectomy. Imaging analysis could not be performed in 62 patients for following reasons: there was no preoperative MRI (n = 32), the preoperative MRI was performed at another hospital (n = 18), DWI was not included in the preoperative MRI (n = 6), the ADC value could not be measured due to little residual pancreatic parenchyma (n = 5), and in- and opposed-phase chemical shift images were not included in the MRI (n = 1). Ultimately 83 patients (50 men and 33 women) with a mean age of 66.6 ± 10.3 years old (range, 34-89) were enrolled in this study. PPPD, Whipple’s operation, and distal pancreatectomy were performed by an expert surgeon in 67, 11 and 5 patients, respectively. The indications for surgery included pancreatic cancers (n = 41), common bile duct cancers (n = 30), cystic tumors of the pancreas (n = 8), neuroendocrine tumors (n = 2), pancreatic duct stricture (n = 1), and duodenal cancer (n = 1).
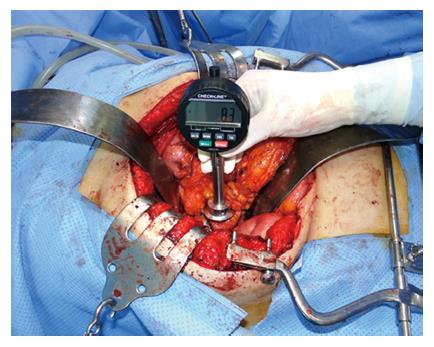
The surgeon who performed the operation subjectively evaluated the pancreatic hardness by palpation during the operation before resection of the pancreas. The pancreatic hardness was classified into the following four categories: very hard, hard, soft or very soft. Objective measurement of the pancreatic hardness was performed by the same surgeon during the operation using a durometer. A Rex Durometer (Rex Gauge, Buffalo Grove, IL, United States) was placed perpendicular to the pancreatic parenchyma where no tumor was located (Figure 1). The unit of the durometer result was displayed using a 0- to 100-point scale in durometer units (DU).
All MRI examinations were performed using a 3-T MR unit (Magnetom Verio; Siemens Healthcare, Erlangen, Germany) with an 8-channel phase-array coil. The detailed parameters of the routine MRI protocol are summarized in Table 1. MR examination consisted of fat-suppressed T2-weighted images, T1-weighted images, chemical shift in- and opposed-phase images, contrast-enhanced T1-weighted images with fat suppression and diffusion-weighted images.
| 3-T system | |||
| T2WI HASTE | T1WI | DWI | |
| TR | 800 | 3.2 | 4500 |
| TE | 95 | 1.3 | 56 |
| ETL | 70 | 1 | |
| Thickness (mm) | 6 | 2.8 | 6 |
| Slice gap | 0 | 0 | 0 |
| FOV (mm²) | 380 × 309 | 380 × 309 | 400 × 313 |
| Matrix size | 320 × 156 | 384 × 250 | 120 × 94 |
| NEX | 2 | 1 | 47 |
| b factor (s/mm2) | 0, 500 | ||
A radiologist with 7 years of experience performed the image analysis. The ADC value of the pancreatic parenchyma was measured three times using an operator-defined irregular shaped ROI (50-100 mm2) on axial images. The mean and minimum ADC values from the ROI with the smallest standard deviation among the three measurements were used for analysis. Estimation of the fat content in the pancreas was performed using a previously suggested method called relative signal intensity decrease (RSID)[13,15]. The signal intensity (SI) of the spleen was used to normalize the pancreatic SI. The RSID was calculated using the following formula: RSID = 100 × (Pin/Sin - Pop/Sop)/(Pin/Sin). Pin and Pop were the signal intensities of the pancreas during in-phase and opposed-phase imaging, respectively. Sin and Sop were the signal intensities of the spleen during in-phase and opposed-phase imaging. The diameters of the pancreatic duct and pancreatic parenchyma at the body-to-tail junction were measured to evaluate the severity of pancreatic atrophy, and the ratio of duct to parenchyma was calculated.
The statistical review of the study was performed by a biomedical statistician. Statistical analysis was performed using SPSS 24.0 (IBM Corporation, Armonk, NY, United States). A P value < 0.05 was considered statistically significant.
Patients were classified into two groups as having a soft or hard pancreas according to the surgeon’s subjective assessment of pancreatic hardness. Both very hard and hard pancreases were included in the hard pancreas group, while both soft and very soft pancreases were included in the soft pancreas group. The durometer measurement, ADC value, RSID, ductal diameter, parenchymal diameter and duct/parenchyma ratio (D/P ratio) were compared between the soft and hard pancreas groups using the Mann-Whitney U test. The Spearman correlation test was used to evaluate the correlation between the ADC value, RSID, diameters of the pancreatic parenchyma and pancreatic duct or D/P ratio and the durometer measurement. The association of each variable with subjective pancreatic hardness was evaluated using univariate logistic regression analysis. Variables with statistical significance or borderline significance (P < 0.15) were used in the multivariate logistic regression analysis.
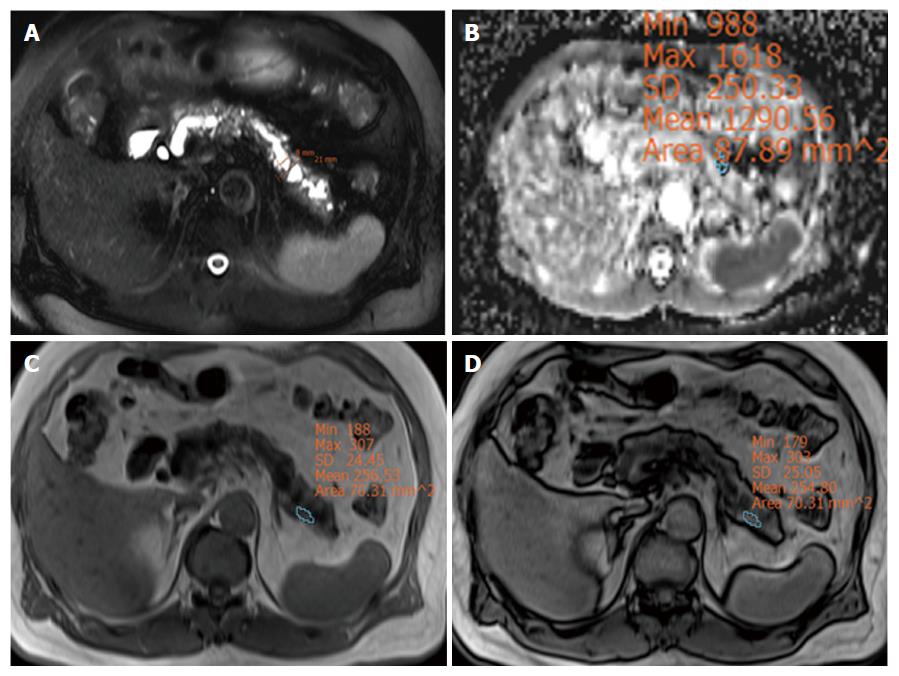
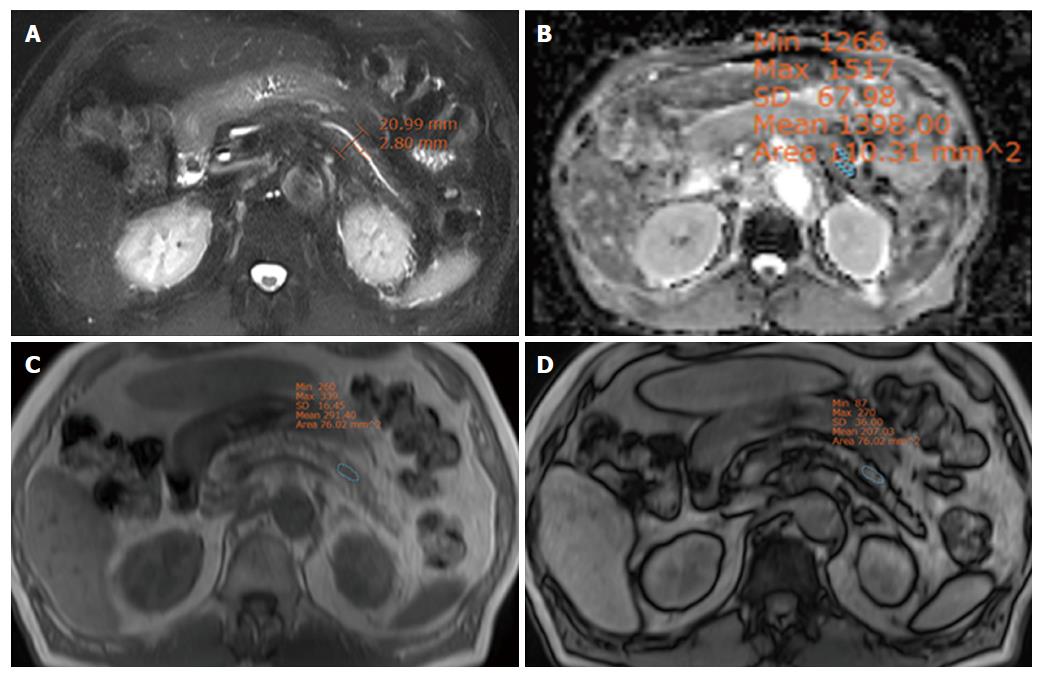
The surgeon classified 39 patients as having a hard pancreas and 44 patients as having a soft pancreas. The duct diameter and D/P ratio in the hard pancreas group were significantly greater than in the soft pancreas group (P < 0.001). The mean and minimal ADC values in the hard pancreas group were significantly lower than in the soft pancreas group (P = 0.012, 0.004, respectively) (Table 2). The RSID was not different between two groups. Two representative cases are displayed in Figures 2 and 3.
| Soft (n = 44) | Hard (n = 39) | P value | |
| Parenchyma diameter | 15 (13-20.75) | 15 (13-20) | 0.791 |
| Duct diameter | 3 (1-3) | 5 (3-7) | < 0.001 |
| D/P ratio | 0.13 (0.08-0.23) | 0.25 (0.18-0.55) | < 0.001 |
| ADCmean (mm2/s) | 1.62 (1.50-1.77) | 1.45 (1.24-1.63) | 0.012 |
| ADCmin (mm2/s) | 1.51 (1.32-1.59) | 1.27 (1.11-1.47) | 0.004 |
| RSID (%) | 10.2 (5.9-22.5) | 15.2 (7.8-22.8) | 0.174 |
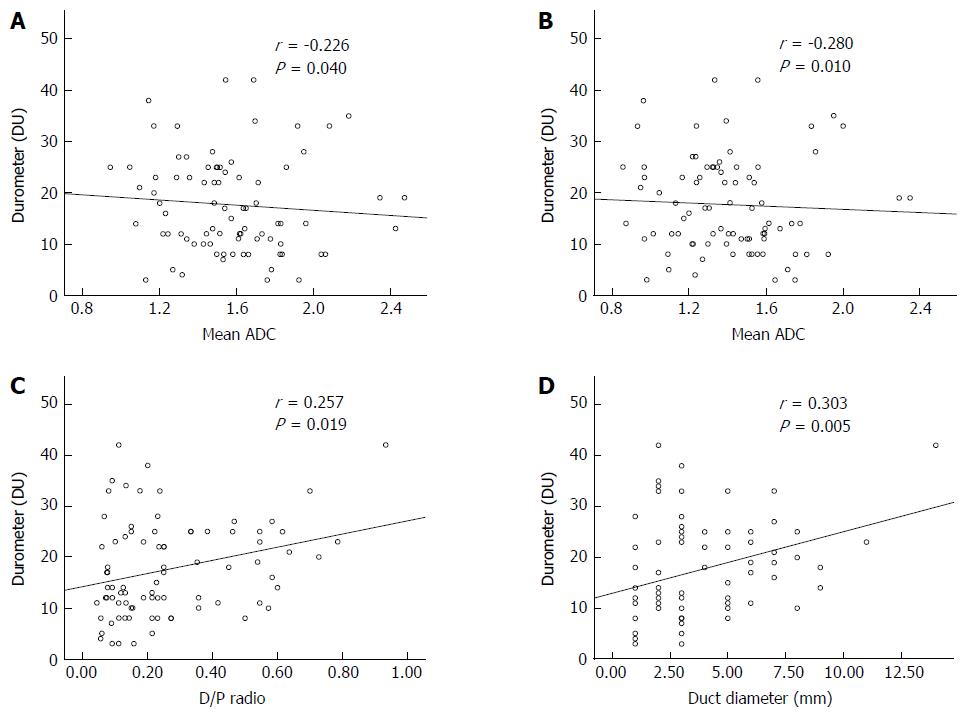
The durometer measurement was significantly lower in the soft pancreas group [median 11, interquartile range (IQR) 8-13] than in the hard pancreas group (median 25, IQR 21-28) (P < 0.001). The mean and minimal ADC were negatively correlated with durometer measurement, and the correlation was statistically significant (P < 0.040, r = -0.280 and P < 0.010, r = -0.223, respectively). The ductal diameter showed a significant correlation with the durometer measurement (P < 0.005, r = 0.303). There was also significant correlation between the D/P ratio and the durometer measurement (P < 0.019, r = 0.257) (Figure 4). The diameter of the pancreatic parenchyma and RSID were not correlated with the durometer measurement (P = 0.724, r = 0.039 and P = 0.052, r = 0.214, respectively).
Univariate logistic regression analysis showed that duct diameter, D/P ratio, and ADCmin were the factors that were significantly associated with subjective pancreatic hardness. Because the durometer measurement is an objective measurement of the hardness of the pancreas, it was excluded from the regression analysis. These three significant factors and ADCmean, which had borderline significance in the univariate analysis, were used in the multivariate regression analysis. Only ductal diameter remained significant after multivariate regression analysis. These data are summarized in Table 3.
| Univariate | Multivariate | |||||
| Coefficient | SE | P value | Coefficient | SE | P value | |
| Parenchyma diameter | -0.022 | 0.044 | 0.611 | - | ||
| Duct diameter | 0.520 | 0.137 | < 0.001 | 1.001 | 0.380 | 0.008 |
| D/P ratio | 4.819 | 1.442 | 0.001 | -3.616 | 4.102 | 0.378 |
| ADCmean (mm2/s) | -0.001 | 0.001 | 0.083 | 0 | 0.003 | 0.923 |
| ADCmin (mm2/s) | -0.002 | 0.001 | 0.017 | -0.004 | 0.003 | 0.166 |
| RSID (%) | 0.013 | 0.014 | 0.356 | - | ||
In this study, three methods to measure the hardness of the pancreas, including radiologic findings, durometer measurements and surgeon palpation, were well correlated with each other. Given that the durometer measurement during the operation correlated well with the surgeon’s subjective measurement, the durometer could be considered a good objective measurement method. Among the studied radiological factors, ductal diameter was the factor that most highly correlated with durometer measurement. The mean and minimal pancreatic ADC values were negatively correlated with durometer measurement to a statistically significant extent. Therefore, preoperative radiologic evaluation can be useful to predict the texture of the pancreas.
The single radiological factor that remained associated with pancreatic hardness after multivariate regression was ductal diameter. More fibrosis of the pancreas makes the pancreas harder, and duct dilatation is aggravated as the pancreas atrophies and fibrosis progresses[16]. In our study, the diameter of the pancreatic parenchyma was not correlated with durometer measurement. Therefore, dilatation of the duct is more important to predict fibrosis of the pancreas. As post pancreatectomy fistulae develop less frequently in fibrotic pancreases, duct dilatation may be a predictive marker for pancreatic hardness and risk of postoperative fistula[6].
There have been several studies regarding the prediction of the texture of the pancreas using preoperative MRI. Advanced MR techniques, such as intravoxel incoherent motion (IVIM) and MR elastography (MRE), have been applied to evaluate pancreas stiffness[17,18]. However, we used more common techniques, including DWI and in- and opposed-phase chemical shift images, because such emerging techniques are not generally available at all institutions. Additionally, the unique feature of our study is the correlation between the MRI measurements and the durometer measurements, which is the more intuitive and direct parameter for the hardness of the pancreas. Our results that the duct diameter and ADC were well correlated with the durometer measurement may be more useful clinically.
In this study, the ADC value was significantly correlated with durometer measurement. The ADC value was independently associated with pancreatic fibrosis and pancreatic stellate cell activity, which is known to produce desmoplasia in chronic pancreatitis and pancreas cancer[10,11]. Considering pancreatic hardness is associated with fibrosis, a low ADC value due to a hard texture can be explained by the decreased diffusion of water and the high density of fibrin within the tissue. The usefulness of the ADC to evaluate for fibrosis or hardness of the tissue has already been proven in other organs such as the liver and kidney[19-21]. In our study, the minimal ADC value showed a higher degree of correlation than the mean ADC value. As the minimal ADC value indicates the most severe diffusion restriction, this measurement may represent the severity of the fibrosis more accurately than the mean ADC value.
Pancreatic hardness can be associated with fibrosis and fat content. In a previous study, the histological fibrosis stage but not the histological fat fraction differed among groups with different intraoperative pancreatic textures. However, the fat fraction on MRI was a significant factor that correlated with advanced fibrosis of the pancreas, and the fat fraction increased as the degree of fibrosis rose. Furthermore, the fat fraction on histologic examination was not correlated with a subjective assessment of pancreatic texture[18]. Our study showed similar results, and the RSID tended to be higher in the hard pancreas group than in the soft pancreas group, though the difference was not statistically significantly different. A larger proportion of fat in the tissue causes a greater signal decrease in opposed-phase compared with in-phase imaging; thus, a higher RSID means more fat in the tissue. Based on these results, we assumed that the fat fraction of the pancreas may be elevated with fibrosis and that the fat fraction may not independently affect pancreatic hardness.
There are several limitations in this study. First, advanced radiologic techniques such as IVIM and MRE were not applied. As they are relatively new and highly costly, they are not commonly used in many hospitals. Therefore, we thought that generally available techniques including DWI and chemical shift imaging were more useful clinically. Second, image analysis was performed by one radiologist, and the durometer measurements were performed by one surgeon. We could not evaluate the reproducibility of these radiologic and durometric analyses. However, repeated durometer measurement during the operation to evaluate reproducibility was impossible as we did not want to prolong operation time any more than strictly necessary. As the radiologic and durometric assessments were performed by experts, we believe that the reproducibility of the measurement was sufficiently high. Third, considering the retrospective nature of this study, selection bias cannot be avoided. Furthermore, many candidates were not enrolled because of the lack of MRI data. However, we enrolled almost all patients who had data for durometer measurement and MRI to tried to overcome this shortcoming.
In conclusion, hard pancreases showed lower ADC values, wider pancreatic duct diameters and higher duct-to-pancreas ratios than soft pancreases. Additionally, the ADC value, pancreatic duct diameter and duct-to-pancreas ratio also correlated well with the durometer results.
The texture of the pancreas is an important predictive factor for the development of postoperative complications following pancreatic surgery. Postoperative pancreatic leakage or fistula formation is more frequent in soft pancreases than hard pancreases. A hard pancreas resulting from fibrosis has a high suture-hold capacity and low pancreatic juice secretion; thus, pancreatic leaks or pancreatic fistulae occur less frequently in patients with a hard pancreas. A decreased pancreatic fat content and increased fibrosis are related to a harder pancreatic texture. The most commonly used method to assess pancreatic hardness in practice is the surgeon’s subjective determination of pancreatic hardness during the operation. Durometric measurements correlated well with surgeons’ palpation-based assessment and histologic evaluation for fibrosis and fat content. Magnetic resonance imaging (MRI) is one possible candidate for the preoperative evaluation of the texture of the pancreas. Diffusion-weighted imaging (DWI) can be applied to the pancreas to measure pancreatic fibrosis
Because surgical resection is the only treatment expecting cure in pancreatic cancer, expanding indications of pancreatic surgery and reducing its complication is very important and many researches are published recently.
Hard pancreases showed lower apparent diffusion coefficient (ADC) values, a wider pancreatic duct diameter and a higher duct-to-pancreas ratio than soft pancreases. Additionally, the ADC values, diameter of the pancreatic duct and duct-to-pancreas ratio were closely correlated with the durometer results. Therefore, hardness of pancreas could be predicted preoperatively with MRI, which may be helpful surgeons to reduce surgery-related complications. However, there was no study correlating the hardness of pancreas to MRI findings.
If MRI is useful to predict the hardness of pancreas, it can help surgeons to reduce surgery-related complications. The authors expect future researches related to the complication rate of pancreatic surgery to the measurements from durometer and MRI.
DWI is an imaging method that uses the diffusion of water molecules to generate contrast in MR images. It allows the mapping of the diffusion process of molecules, mainly water, in biological tissues, in vivo and non-invasively. ADC is a measure of the magnitude of diffusion (of water molecules) within tissue, and is commonly clinically calculated using MRI with diffusion weighted imaging. In- and opposed-phase: Because water and fat protons have slightly different resonance frequencies, their spins go in- and out-of-phase with each other as a function of time. On in-phase image, signals from water and fat are in the same direction and the signal is addition of these two signals. However, on opposed-phase, signals from water and fat are in the opposite direction and cancel each other.
This work is a review on assessing the pancreatic hardness by examining the pancreas using the durometer and MRI. Eighty three patients were involved in this study and the authors concluded that hard pancreases showed lower ADC values which correlated with the durometer results. This work has adequate number of patients and an error analyses. It is very well prepared and the conclusion is useful for the reader.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chow J S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, Traverso LW. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451-1458; discussion 1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Hamanaka Y, Nishihara K, Hamasaki T, Kawabata A, Yamamoto S, Tsurumi M, Ueno T, Suzuki T. Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery. 1996;119:281-287. [PubMed] [Cited in This Article: ] |
| 3. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Belyaev O, Munding J, Herzog T, Suelberg D, Tannapfel A, Schmidt WE, Mueller CA, Uhl W. Histomorphological features of the pancreatic remnant as independent risk factors for postoperative pancreatic fistula: a matched-pairs analysis. Pancreatology. 2011;11:516-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Sandini M, Malleo G, Gianotti L. Scores for Prediction of Fistula after Pancreatoduodenectomy: A Systematic Review. Dig Surg. 2016;33:392-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Belyaev O, Rosenkranz S, Munding J, Herzog T, Chromik AM, Tannapfel A, Uhl W. Quantitative assessment and determinants of suture-holding capacity of human pancreas. J Surg Res. 2013;184:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Crippa S, Salvia R, Falconi M, Butturini G, Landoni L, Bassi C. Anastomotic leakage in pancreatic surgery. HPB (Oxford). 2007;9:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Belyaev O, Herden H, Meier JJ, Muller CA, Seelig MH, Herzog T, Tannapfel A, Schmidt WE, Uhl W. Assessment of pancreatic hardness-surgeon versus durometer. J Surg Res. 2010;158:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Tanaka K, Tomita H, Osada S, Watanabe H, Imai H, Sasaki Y, Goshima S, Kondo H, Kanematsu M, Hara A. Significance of histopathological evaluation of pancreatic fibrosis to predict postoperative course after pancreatic surgery. Anticancer Res. 2015;35:1749-1756. [PubMed] [Cited in This Article: ] |
| 11. | Watanabe H, Kanematsu M, Tanaka K, Osada S, Tomita H, Hara A, Goshima S, Kondo H, Kawada H, Noda Y. Fibrosis and postoperative fistula of the pancreas: correlation with MR imaging findings--preliminary results. Radiology. 2014;270:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Balci NC, Perman WH, Saglam S, Akisik F, Fattahi R, Bilgin M. Diffusion-weighted magnetic resonance imaging of the pancreas. Top Magn Reson Imaging. 2009;20:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Yokoyama Y, Ebata T, Igami T, Sugawara G, Ando M, Nagino M. Proposal for a Pancreatic Configuration Index for Determining Patients at High Risk of Pancreatic Fistula Following Pancreatoduodenectomy. Dig Surg. 2016;33:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kim SH, Lee JM, Han JK, Lee JY, Lee KH, Han CJ, Jo JY, Yi NJ, Suh KS, Shin KS. Hepatic macrosteatosis: predicting appropriateness of liver donation by using MR imaging--correlation with histopathologic findings. Radiology. 2006;240:116-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Balci NC, Bieneman BK, Bilgin M, Akduman IE, Fattahi R, Burton FR. Magnetic resonance imaging in pancreatitis. Top Magn Reson Imaging. 2009;20:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging. 2015;41:369-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ, Jang JY, Kannengiesser S, Han JK, Choi BI. Pancreatic Steatosis and Fibrosis: Quantitative Assessment with Preoperative Multiparametric MR Imaging. Radiology. 2016;279:140-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Jiang H, Chen J, Gao R, Huang Z, Wu M, Song B. Liver fibrosis staging with diffusion-weighted imaging: a systematic review and meta-analysis. Abdom Radiol (NY). 2017;42:490-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Hueper K, Khalifa AA, Bräsen JH, Vo Chieu VD, Gutberlet M, Wintterle S, Lehner F, Richter N, Peperhove M, Tewes S. Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J Magn Reson Imaging. 2016;44:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Zhao J, Wang ZJ, Liu M, Zhu J, Zhang X, Zhang T, Li S, Li Y. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol. 2014;69:1117-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |