Abstract
Recently, an expanding body of knowledge has documented the nature and functions of receptors in joint tissues and their potential importance in preserving the smooth normal functioning of the motor-skeletal system and in amplifying the inflammatory response to joint injuries and diseases. This review summarizes the current knowledge of the anatomical and physiological substrates of these mechanisms. The distribution, morphologic and functional characteristics of joint receptors have been well described. In the past decade there has been a new appreciation of the major role played by sensory neurons in promoting regional inflammatory responses, and many of the specific neuronal mechanisms and molecules that mediate these reflexes have been identified. This knowledge promises to significantly improve the selectivity and effectiveness of pharmacologic approaches to pain, trauma and regional inflammatory disorders.
Other investigations have revealed important contributions of joint receptors to motor function. These refer not to proprioception or the sense of limb position in space, but rather to a more sophisticated tailoring of muscle activity to increase joint stability and to protect joint structures from damaging loads. Whether a loss of these reflexes may play a role in the pathogenesis of osteoarthritis remains controversial. However, there is a growing consensus that a loss of these reflexes may contribute to the morbidity associated with disruption of the anterior cruciate ligament.
Synovial joints are sites of major interactions between the musculoskeletal and the nervous systems. Understanding the mechanisms that activate and control these interactions will certainly offer the opportunity to develop new, more effective treatments for patients with joint disorders.
A primary function of the musculoskeletal system is to act as an effector system for the central nervous system, thereby providing an organism with the enormous advantage of an ability to purposefully manipulate the environment. It is sensible that a mechanism to monitor performance should be intrinsic to such a system, which is a teleologic explanation for the existence of specialized sensory receptors within muscle and joint tissues. Extensive information has accrued regarding the length-and tension-sensing functions of muscle spindle receptors and Golgi tendon receptors. In recent years an expanding body of knowledge has documented the nature and functions of receptors situated in joint tissues and their potential importance in preserving the normal functioning of the motor-skeletal system and in amplifying the inflammatory response to joint injuries and diseases.
Anyone who has experienced the pain and disability of a joint injury or inflammatory condition has first-hand knowledge of the potent reflex mechanisms mediated by joint receptors to protect an injured joint from further damage by activating the dynamic splinting and guarding effects of muscle spasm or the withdrawal response to pain. To what extent do protective neuronal reflexes actively participate in the generation and perpetuation of an inflammatory response? And are there other related reflexes that function routinely at an unconscious level to protect healthy joints from damage during normal activity?
Articular neuroanatomy
A majority of investigations of joint innervation have focused on the knee joint. The vertebrate knee joint is supplied by 2 major articular nerves — the posterior articular nerve, which is a branch of the tibial nerve, and the medial articular nerve, which arises from the femoral or obturator nerve. Together these account for 80% to 90% of the neurons supplying the joint. Hilton proposed that joints receive some branches from all the nerves that supply muscles which traverse the joint; however, in the knee joint such accessory contributions seem to be variable, inconsistent and often absent.1,2
All articular structures except articular cartilage are innervated.3 Joint nerves are composed of both myelinated and unmyelinated fibres. About 20% of the axons are myelinated. They contain large diameter mechanoreceptors, which are thickly myelinated and are rapid conductors, and smaller diameter, thinly myelinated nociceptors and mechanoreceptors. About 80% of the axons are unmyelinated, half are sympathetic fibres and the rest are sensory fibres.4,5
Joint tissues contain a variety of sensory nerve endings, which can be broadly classified into 4 morphologic types (Table I). Three types are corpuscular mechanoreceptors and the fourth type, which is by far the most numerous, is the free nerve endings. Table I lists the types of receptors together with their typical locations in the cat knee and gives a simplified description of their functional characteristics. There seem to be some minor variations in receptor morphology across species, but these variations are unlikely to be responsible for significant functional differences.6–8 In general terms, these receptors respond to tension, pressure or noxious stimuli. There is likely some overlap of functions between receptor types; for example, some slowly conducting unmyelinated fibres are known to be responsive to mechanical stimuli.9
Classification of Joint Receptors in a Cat Knee*
Neurons that innervate joints and their role in arthritis
Clinical observations indicate the existence of important interactions between the nervous system and joints involved by arthritis or injury. For example, rheumatoid arthritis typically occurs in a symmetrical pattern of joints, except in patients with focal neurologic disease, who may show sparing of paralysed or denervated joints.10,11 Reflex dystrophy syndrome is often accompanied by synovitis. A significant component of the disability accompanying disruption of the anterior cruciate ligament may be caused by the loss of proprioceptive mechanisms mediated by mechanoreceptors in the ligament.6,12,13 Over the past decade there has been a remarkable increase in understanding the neural substrates of these clinical phenomena.
It is helpful to classify arthritis into 2 broad categories: inflammatory and degenerative. In inflammatory arthritis, the essential pathophysiology is the development of synovitis, with damage to joint soft-tissue constraints (joint capsule, ligaments and meniscal structures) and cartilage matrix occurring secondary to the actions of enzymes, cytokines, peptides, prostaglandins, free radicals and other mediators that are liberated by the inflammatory process. In degenerative arthritis, cartilage damage may result from excessive loading of normal cartilage or may reflect an intrinsic mechanical inadequacy of abnormal cartilage matrix that is unable to withstand normal loads. The role of joint-receptor-mediated reflexes in these 2 categories of arthritis will be considered.
Neuronal contributions to inflammatory arthritis
In 1937, Sir Thomas Lewis first postulated the existence of a “nocifensor system” of nerve fibres in skin, based primarily upon his observations of the cutaneous triple response: red line, flare and weal. This system, consisting of neurons coursing through the dorsal root ganglia, was thought to release mediators in the region of a noxious stimulus by the generation of antidromic impulses stimulated by the “axon reflex.”14 The proposed function of these reflexes was the regionally selective promotion and amplification of the inflammatory response, the activation and facilitation of an appropriate local defence or a repair response after a noxious or damaging insult.
Over the past 20 years significant advances in our understanding of these reflex mechanisms have been made, together with the realization that they likely play an intrinsic role in all inflammatory conditions. Nowhere has this become more evident than in joints. Inflammatory arthritis induces profound changes in sensory neurons at their peripheral terminations, in their cell bodies in the dorsal root ganglia, in their spinal cord connections and even in the neurons that supply the contralateral joint.
Peripheral neurogenic mechanisms in inflammatory arthritis
Role of neuropeptides
About one-third of the unmyelinated free nerve endings in joints contain substance P or calcitonin gene-related peptide (CGRP), or both,15,16 peptides that are potent inflammatory mediators. Substance P is a vasodilator, which increases capillary permeability, induces mast cell de-granulation and is a potent leukocyte chemotactic agent.17,18 It is also mitogenic and chemotactic for endothelial cells and fibroblasts. CGRP is a potent vasodilator, a mitogen for endothelial cells and a potent inhibitor of insulin-mediated glycogen synthesis. 19 Acting together, these neuron-derived peptides can significantly potentiate the inflammatory response, and they are released into the joint and peri-articular tissues during inflammatory arthritis.20
Tissue injury increases the local expression of nerve growth factor by fibroblasts in response to tumour necrosis factor-α or interleukin-1β.21,22 Levels of nerve growth factor in synovial fluid are significantly increased in patients with inflammatory arthritis. 23 Nerve growth factor is a trophic factor for mast cells, small diameter peptidergic neurons and sympathetic efferent neurons. Substance P and CGRP messenger RNA (mRNA) and peptide content in the dorsal root ganglia and dorsal horn of the spinal cord are increased dramatically after induction of experimental inflammatory arthritis as a result of increased expression of nerve growth factor in the damaged target tissue.17,24–29
Two classes of peripheral nociceptor
About one-third of unmyelinated sensory fibres do not express substance P or CGRP. Recent investigations of these neurons have revealed enough characteristic differences to suggest that they should be considered in a separate functional class. They express a receptor for a different neurotrophic factor called glial-derived neurotrophic factor and also selectively express receptors for adenosine triphosphate that are not found on neuropeptide-containing neurons. Furthermore, this subgroup of unmyelinated sensory neurons exhibits a different pattern of axon termination in the dorsal horn of the spinal cord.30,31 The significance of these differences remains to be determined, but there is a clear indication that these different neuronal subtypes may mediate different types of pain and may therefore be selectively blocked by appropriate ligands.
Sensitization of joint afferent neurons during inflammation
Pain is one of the cardinal signs of arthritis and is usually associated with allodynia (pain to innocuous stimuli) and hyperalgesia (amplified pain to noxious stimuli). These perceptual changes are now known to reflect cellular and molecular changes in the cells of the dorsal root ganglia and the spinal cord. Allodynia and hyperalgesia come about because of changes that originate almost exclusively in the smaller diameter thinly myelinated and unmyelinated populations of sensory fibres. These neurons exhibit enlargement of their receptive fields and decreased sensory thresholds to mechanical and noxious stimuli.4 Many neurons begin to fire spontaneously, and others fire at much higher rates in response to stimulation. Although many of the well-known inflammation-associated mediators (e.g., bradykinin, serotonin, prostaglandins) have been proposed as causes of or contributors to these changes in neuronal response properties, it was recently shown that almost all of the observed changes in the behaviour of these neurons may be induced by locally increased concentrations of nerve growth factor.32–34
Role of autonomic fibres
Vasomotor reflexes become significantly altered during the inflammatory response to joint inflammation. Normal joint tissues have a relatively low baseline blood flow, which can be modulated by stimulation of sympathetic efferents or application of substance P and CGRP.35,36 These reflexes are abolished by adjuvant arthritis, which induces significant increases in joint blood flow that can no longer be altered by sympathetic stimulation or the application of neuropeptides.37
Sympathetic fibres make a contribution to plasma extravasation. Increased tissue levels of bradykinin induce the release of serotinin (5-hydroxytryptamine) from mast cells and platelets. Serotonin mediates plasma extravasation from the synovial microcirculation by actions on 5-hydroxytryptamine-2A receptors that are found on sympathetic efferent terminals. It is thought that activation of the 5-hydroxytryptamine-2A receptor induces release of prostaglandins from the sympathetic terminal by a mechanism independent of action potentials or membrane depolarization. These effects are blocked by sympathectomy or selective antagonists at the 5-hydroxytryptamine-2A receptor.38
Spinal reflexes regulate the regional inflammatory response
The severity of experimentally induced inflammatory arthritis of the rat knee joint can be significantly diminished by dorsal root transection, disconnecting the dorsal root ganglion from the spinal cord, but leaving intact the axons connected to the periphery. 39 This seminal observation indicates that a spinal cord circuit must mediate most of the release of the neuropeptides that amplify the inflammatory response.
According to this model, afferent volleys are carried by myelinated and unmyelinated nociceptive neurons into the spinal dorsal horn, where they synapse on second order neurons and interneurons.40 The second order neurons or interneurons then activate other peptidergic neurons to antidromically conduct impulses back to the periphery, where substance P, CGRP and other neuromodulators are released. Although the precise spinal circuitry remains unknown, follow-up experiments have shown that these “dorsal root reflexes” utilize glutamate and γ-aminobutyric acid (GABA) as transmitters and can be blocked by selective antagonists at the non-N-methyl-d-aspartate glutamate receptor and the GABAA receptor.41,42
Sensitization of intraspinal second order sensory neurons
Dorsal horn neurons of the spinal cord receiving input from joints may be classified into 2 types: nociceptive specific and wide dynamic range. Nociceptive-specific neurons respond only to noxious or damaging stimuli. Wide-dynamic-range neurons show a graded response to various stimuli, with very high outputs in response to noxious stimuli.9 Just as inflammation-associated allodynia and hyperalgesia arise from lowered thresholds of primary afferent neurons, these sensory disturbances also reflect significant increases in excitability of the both the wide-dynamic-range and nociceptive-specific types of second order sensory neurons in the spinal cord that show lowered thresholds, higher firing rates and sometimes spontaneous discharges. In recent years, the causes of these changes in excitability have been elucidated. Increased sensory traffic from the inflamed joint leads to localized increases in dorsal horn levels of glutamate, substance P and CGRP. Hyperexcitability of the second order neurons deep in the dorsal horn is principally caused by the actions of glutamate upon metabotropic glutamate receptors43 and of GABA (presumably released from interneurons) upon the GABAA receptor41 but is further augmented by the actions of intraspinal substance P and CGRP.44,45 Blockade of the metabotropic glutamate receptor or the GABAA receptor can prevent the development of hyperexcitability of the second order sensory neurons in the spinal cord without affecting normal responses to innocuous and noxious stimuli.41,43
Recent experiments have revealed that an experimental inflammatory arthritis can also increase substance P and CGRP production in the dorsal root ganglia and spinal cord contralateral to the inflamed joint and even induce a symmetrical inflammation in the contralateral joint.46,47 These observations lend further support to the evidence that afferent and spinal mechanisms play a key role in the initiation and promotion of inflammation. These “contralateral effects” can be prevented by blockade of the “dorsal root reflexes” with appropriate antagonists to glutamate and GABA at the appropriate receptors.
Protective effects of joint afferent and efferent neurons
The inflammatory process plays important roles in defending against infection and in initiating repair mechanisms after injury. Our current understanding of the neurogenic contribution to inflammation fulfils the hypothetical role postulated by Sir Thomas Lewis for his “nocifensor” system. Furthermore, it has recently been shown that local immune responsiveness is increased by a glutamate-mediated spinal reflex.48 The mechanism by which this occurs remains obscure. It also remains to be seen whether neurogenic inflammatory mechanisms have any significant influence on the outcome of tissue repair.
Clinical relevance
These recent findings are of broad clinical interest because they reawaken an interest in a previously under-appreciated and potentially significant capability of spinal cord and peripheral sensory neurons to regulate specific metabolic processes in a regionally selective manner. Targetting the upregulation of nerve growth factor in injured tissues or perhaps the specific neurotransmitter receptors on second order neurons in the spinal cord could realistically lead to the development of more effective drugs and treatment for trauma, postoperative inflammation and other forms of chronic arthritis in which inflammation contributes significantly to morbidity.
Neurologic factors in degenerative arthritis
Charcot’s arthropathy
Charcot’s arthropathy is a severe form of degenerative arthritis that occurs in patients with peripheral sensory neuropathy.49–52 Although Charcot believed the disease was caused by a loss of trophic substances derived from the nerves, leading to atrophy of the joint tissues, the views of Volkmann have subsequently dominated modern thinking.51 Volkmann and later Virchow argued that the loss of limb sensation compromised muscular function in the limb, leading to mechanical damage to the joint.53 This became the consensus opinion of subsequent investigators. Although there has been considerable speculation, the potential relationship of the pathogenic mechanisms of Charcot’s arthropathy to the common forms of osteoarthritis remains controversial.12,13,54,55
Osteoarthritis, aging and joint innervation
Osteoarthritis is one of commonest disabling conditions associated with aging. The prevalence of osteoarthritis correlates directly with age and shows an increase with every decade after the age of 25 years. Age is the highest risk factor for osteoarthritis,56 and 27%, 34% and 44% of the population exhibit radiographic osteoarthritis of the knee in the seventh, eighth and ninth decades of life respectively. 57,58 Yet the normal aging of articular cartilage and subchondral bone does not presage the pathological changes found in osteoarthritis,56 and the underlying cause of the disorder remains unknown. As Brandt59 suggested, the end stage of osteoarthritis represents “joint failure,” but the primary defect may lie in cartilage, synovium, subchondral bone, ligament or the neuromuscular system. Indeed, it has been repeatedly suggested that a failure of protective neuromuscular reflexes could predispose people to osteoarthritis. 60–62
A number of investigations in humans have hinted that neural mechanisms may play an important protective role in normal joint function. For example, a study using metal-impregnation stains to examine biopsy specimens of human knee joint capsule and ligament obtained from elderly patients with osteoarthritis at the time of joint replacement surgery failed to detect any of the neural elements that could be found in newborn or adolescent tissue.63 Another more recent study identified a group of seemingly normal subjects with very subtle deficiencies in motor coordination during walking gait, manifested by abnormal peak loading during the early stance phase.62 This subgroup of “normal” people might be particularly dependent on joint afferent systems to protect their joints from damaging loads or might even have a deficiency of joint afferent innervation. Taken together, these studies lend support to speculations that an age-related loss of joint sensory innervation could cause or accelerate the development of common human osteoarthritis. 13,60,61
Neurosensory function of ligaments
In recent years these speculations have been fuelled by experiments that found that simple knee joint denervation in dogs produced minimal to no cartilage degeneration at up to 16 months. However, if denervation was combined with transection of the anterior cruciate ligament, then an extremely rapid and progressive knee joint degeneration did ensue.64,65
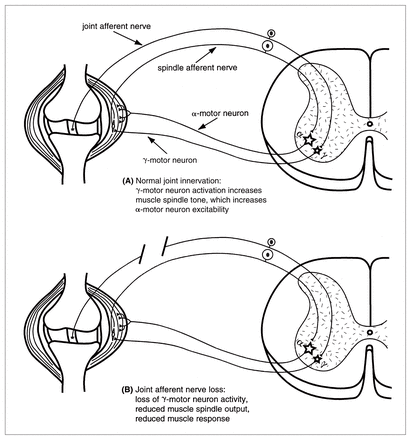
These observations may be partially explained by electrophysiological studies in cats that have clearly established the existence of the neural circuitry necessary to allow joint afferent neurons to mediate functionally important motor reflexes that are appropriate to protect joint surfaces from damaging loads.12,13,54,66–72 Briefly, ligament-associated mechanoreceptors have been found to have a major influence on muscle spindle activity by regulating γ-motor neuron activation of the intrafusal muscle fibres. Stimulation of receptors in the cruciate ligaments, for example, increases γ-motor neuron outflow, muscle spindle sensitivity and muscle tension in both the flexor and extensor muscles of the knee joint.69,70,73 The net effect is a reflex increase of limb stiffness in response to applied loads. This balancing of muscle activities acting across the joint increases joint stability, which could be expected to protect the joint surfaces from mechanical damage (Fig. 1).
Diagram of a knee joint and associated spinal segment illustrating how joint receptors function as part of a feedback loop within the γ-motor neuron and muscle spindle receptor feedback loop. (A) Under normal circumstances, ligament stretch increases firing in mechanoreceptors, which increases the γ-motor neuron activity. This, in turn increases muscle spindle sensitivity to stretch, which feeds back on to the α-motor neuron, thereby increasing muscle tension, which increases joint stability. (B) Loss of joint afferent input due to ligament disruption or neuronal loss leads to a reduced γ-motor neuron outflow and ultimately a loss of muscle force, which decreases joint stability.
Age-related loss of mouse knee joint innervation
Discussions about protective muscular reflexes mediated by joint receptors and their relationship to “idiopathic” degenerative arthritis are of less interest, however, unless there is a loss of joint innervation with aging. Age-related loss of neurons from the central nervous system is a well-established phenomenon but has not been widely documented in the peripheral nervous system. Experiments in our laboratory revealed that in mice, innervation of the knee joint can be accurately and consistently quantified by retrograde tracing with the fluorescent dye Fluoro-Gold (Fluorochrome, Inc., Inglewood, Colo.). When this technique was used in C57BL/6 mice of different ages, a 60% loss of joint afferent neurons over the life span of the mouse was found.74 Most of the loss occurs in the first 6 months of the mouse’s 2-year mean life span and precedes the onset of degenerative arthritis, which has been reported to occur in this strain of mice.75,76 Rats are genetically closely related to mice but are rarely afflicted by degenerative arthritis. Interestingly, in experiments using Fluoro-Gold labelling of joint innervation in aging rats I found no losses of joint innervation with aging (unpublished data).
Joint receptor function in humans
Because few investigators studying human specimens have attempted to accurately quantify innervation of the knee joint, it remains unknown whether similar losses can and do occur in humans. However, in a series of studies some have attempted to approach this question indirectly by examining proprioceptive function in the hip and knee joint of awake human subjects. These studies did show some impairment of joint position sense with aging and after ligament injury or joint replacement injury.77–80 However, the relationship of a diminution of the conscious perception of joint position and movement to the type of protective muscular reflexes I have described is questionable at best. It is now clear that the conscious perception of joint position is mediated almost exclusively by muscle spindle receptors.81 The perception of joint or limb position in space is an important and useful sensory function, but it is not the same as the detection and modulation of joint loads, which is an unconscious, reflex motor function. A more useful (although more technically challenging) study of the function of joint receptors and the consequences of their loss would attempt to quantify muscle activities during load-bearing activity. A loss of joint receptor input would be expected to result in a disturbance of the normal balance of muscle forces across the knee joint. Such a study has been done. In 2 recent papers73,82 it was reported that in a group of patients with chronic anterior cruciate ligament injury there were measurable changes in motor function. Patients and uninjured control subjects were tested while performing isokinetic resisted knee flexion and extension. Patients generated significantly lower electromyographic activity and muscle force in the hamstring muscles when compared with normal subjects performing the same exercise or when compared with the patient’s contralateral uninjured limb. Of particular note is that surgical reconstruction of the anterior cruciate ligament did not change these observed differences.73,82 These observations are consistent with our current view of ligament-associated mechanoreceptor function derived from the animal laboratory investigations already described. They lend support to the contention that a major element of ligament function is neurosensory and would appear to indicate that the loss of this function is not adequately addressed by the current standard surgical techniques of ligament reconstruction.
Clinical relevance
A cautious interpretation of the currently available data would suggest that if there is an age-related loss of joint innervation in humans, it could be a contributing factor in the pathogenesis of osteoarthritis. Such a loss would likely be of greater significance in association with some type of injury to the joint. In addition, it is wise to recall that the pathogenesis of osteoarthritis is likely multifactorial. Many extrinsic factors such as body mass, activity levels, limb alignment and soft-tissue constraint are also important determinants of the loading history of articular cartilage surfaces, surfaces that almost certainly vary in their tolerance of load, injury and wear.
Current treatments for anterior cruciate ligament deficiency do not address the potentially important neurosensory properties of the native ligament. This may prove to have a significant impact on the ultimate outcome in these patients.
Future directions
The growth of knowledge of the fundamental workings of the nervous system has been explosive. As our knowledge of the role of neural systems and mechanisms in articular health and disease continues to expand, our capability to intervene effectively will also improve. The recent advances in understanding of afferent and spinal cord mechanisms clearly outline the potential for the development of selective drug therapies that could greatly reduce the pain and inflammation of arthritis or joint injury without interfering with normal sensory functions or tissue repair. If age-related loss of joint innervation does prove to be a contributor to the pathogenesis of osteoarthritis, then our approaches to prevention and treatment would understandably be radically altered. Similarly, increased recognition of the role of ligaments’ sensory properties could lead to changes in operative techniques or to the development of methods to improve the survival and regeneration of damaged receptors.
Acknowledgments
Thanks to Dr. K.W. Marshall for assistance with the illustration and to Dr. R.D. Inman for helpful comments.
Supported by MRC grant MT13161.
- Accepted May 7, 1998.