An infected aneurysm is defined as a localized dilation of an artery resulting from some form of arterial wall injury associated with sepsis that may prompt a slow and insidious course of events or quickly lead to devastating complications. Whereas the wall damage may be owing to embolism, atherosclerosis, congenital defects or trauma, sepsis may be secondary to infected emboli, bacteriemia or contiguous infection. Sepsis alone is unlikely to cause an infected aneurysm.1 We report the case of a patient with an infected abdominal aortic pseudoaneurysm that developed after laparoscopic colorectal surgery.
Case report
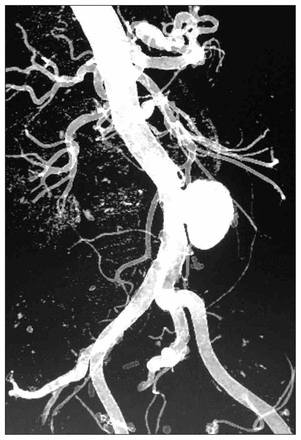
An 86-year-old man presented to our emergency department with a 3-day history of acute abdominal pain 2 months after having laparoscopic surgery for neoplastic disease. An abdominal ultrasound and a computed tomography (CT) scan showed a 25-mm perforation of the middle aorta, with a pseudoaneurysm (Fig. 1). We did not suspect infection (the patient had no fever or leukocytosis), and we repaired the complication with an open prosthetic tube graft insertion that failed 2 weeks later due to disruption of the inferior aortoprosthetic anastomosis. This prompted emergency endovascular aortic repair (EVAR) and femoro–femoral crossover grafting, but the patient returned 20 days later with fever and leukocytosis. A CT scan revealed a painful pseudoaneurysm at the groin and signs of periendoprosthetic infection (Fig. 2). Only bacterial cultures from the wound at the groin proved positive for Pseudomonas aeruginosa. Imaging and laboratory findings were normal following long-term ciprofloxacin therapy, but fever recurred every time the antibiotics were stopped. After discussion among vascular surgeons, vascular radiologists and experts in infectious diseases, it appeared that the best strategy was major open surgery to remove and débride the aorta with endoprosthesis and crossover graft using muscle flaps or the omentum to cover the contaminated field. The patient refused this definitive option given the usually high related risks and mortality. One year later, he remained on antibiotic therapy and was doing well.
A 3-dimensional reconstructed computed tomography scan showing the leakage of the abdominal aortic pseudoaneurysm.
A computed tomography scan showing air bubbles surrounding the prosthetic graft, indicative of graft infection (arrows). The preaortic hematoma has been reabsorbed.
Discussion
The challenges posed by this patient concern 1) the dubious etiology of the aortic wall injury that led to the pseudoaneurysm, 2) the atypical clinical presentation of the aortic complication and 3) the rare type of pathogen causing the infection.
The etiology remains without answer, though we suspected a link between the laparoscopic procedure and the onset of the pseudoaneurysm given the brief time elapsing between the 2 events. However, reviewing the tape of the primary laparoscopic surgery failed to identify the cause of the aortic lesion. The atypical presentation — that the patient oddly had no fever, leukocytosis or other signs of such severe vascular infection — may explain why we did not suspect the infective nature of the pseudoaneurysm and why a diagnosis of iatrogenic aortic injury seemed more likely. It was only when the aortic anastomosis became disrupted 2 weeks after the tube graft was inserted that we suspected a prosthetic infection, but could not confirm it with laboratory and clinical findings. We subsequently chose an EVAR procedure in emergency conditions to repair the ruptured pseudoaneurysm or to attempt a more straightforward procedure to avoid the still-high mortality and morbidity of definitive major open surgery. On the other hand, even though acceptable results have been achieved with aortic endovascular prosthetic grafts used to repair pseudoaneurysms developing where initial open repair has failed,2 using EVAR to treat infected pseudoaneurysms goes against general surgical principles because placing a foreign body in an infected field could exacerbate the infection. The recent literature has nonetheless increasingly emphasized EVAR in such circumstances, although it is still unclear whether this treatment is curative or a bridging procedure.3,4
Aortic endograft infections have reportedly been managed successfully without explanting the graft using specific antibiotic irrigation and protracted intravenous antibiotic therapy, but they were all cases of pathogenic organisms other than P. aeruginosa, which is rarely responsible for arterial infections. The particular characteristics of P. aeruginosa might explain our patient’s persistent infection, recurrent fever and other laboratory data consistent with bacteriemia when specific antibiotic treatment was suspended, despite local signs of his infection disappearing from the CT scan.5
In conclusion, delayed-onset and painful aortic enlargement after laparoscopic surgery should raise the suspicion of an infected pseudoaneurysm, even when laboratory data and clinical signs and symptoms are not consistent with infection. Treatment with EVAR for such severe illness should not be recommended for patients with active infection or infection that is not well controlled by antibiotics; however, it could be used as bridging procedure in such cases, delaying a definitive treatment until the patient’s condition has stabilized.