Unicentric Castleman disease is rare disorder that is characterized by a single tumour, often located in the mediastinum or mesentery. We describe the case of a 29-year-old woman who presented with anemia in whom we found a localized hyaline-vascular subtype tumour, which we resected.
Case report
An asymptomatic 29-year-old woman, with a long-term history of mild anemia, was referred to our hospital for evaluation. She was pale and asthenic.
Except for anemia, her past medical and surgical history was unremarkable. There was no history of spoliation, and we noted no coagulopathy, type-B symptoms or systemic disease. Results of a complete physical examination were normal. The abdomen was soft with no palpable mass.
Laboratory test results showed a decrease in hemoglobin (86 g/L) and mean corpuscular volume (69.9 fL) with thrombocytosis (716 × 109/L). We might have diagnosed iron-deficiency anemia as the patient’s blood iron (2.5 μmol/L) and serum transferrin saturation percentage (0.04) had also decreased, but total iron binding capacity (66.5 μmol/L) and ferritin levels (49 pmol/L) were normal. The erythrocyte sedimentation rate was elevated (116 mm/h), as was C-reactive protein (775.2 nmol/L), accompanied by a mild hypoalbulinemia (34 mmol/L), suggesting a possible inflammatory anemia. Renal and hepatic functions were normal.
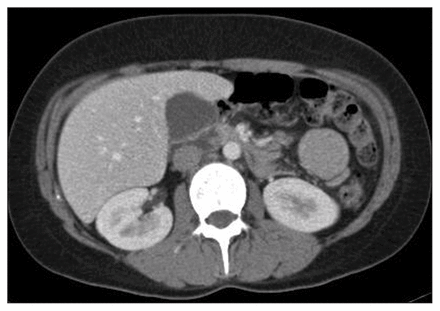
Abdominopelvic ultrasound followed by a computed tomography (CT) scan (Fig. 1) revealed a unique mesenteric, noncalcified contrast-enhancing mass near the jejunum with peripheral vascularity and 3 mesenteric lymph nodes varying from 8 to 12 mm. The mass was capsulated, nonmobile and directly adjacent to mesenteric vessels. We performed surgical excision by laparotomy.
Abdominopelvic computed tomography scan showing a unique mesenteric mass in the jejunal region, noncalcified with regular border, directly on contact with jejunal anses.
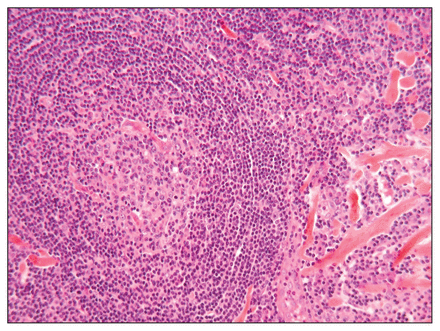
Macroscopic examination revealed a 4.7 × 4.0 × 2.7 cm tumour weighing 27.5 g. The tumour showed lymphoid follicular hyperplasia, fibrotic areas and numerous plasmocytes. Some follicules were surrounded by a lymphocyte crown with a concentric onion-skin appearance, and there was a lymphoplas-mocytic infiltrate with hyaline material between lymphoid nodules (Fig. 2). Immunochemistry analysis showed κ and λ light chains in normal ratios with germinal centres positive for CD20, CD21, CD23, Bcl6 and Ki-67, and negative for Bcl2. The interfollicular spaces were reactive in particular to CD3, CD5, CD20 and Bcl2. These results were compatible with a diagnosis of angiofollicular hyperplasia or unicentric Castleman disease.
Follicular hyperplasia with onion skin appearance and hyaline connective material (hematoxylin phloxine saffron stain, original magnification × 200).
The patient was discharged on the fifth postoperative day after an uncomplicated postsurgical recovery. Her symptoms of inflammatory anemia improved rapidly. Within 2 weeks postoperatively, her hemoglobin level was 105 g/L and mean corpuscular volume was 72 fL; she reached normal values by 3 months postoperatively (hemoglobin 118 g/L). No evidence of recurrence or residual disease appeared on postoperative positron emission tomography and CT scans at follow-up.
Discussion
Castleman disease is a rare hyperplasia of the lymph nodes. This benign disorder is classified as unicentric or multicentric, with hyaline-vascular, plasmacytic or mixed cellularity subtypes. Hyaline-vascular, or angiofollicular, subtypes account for 90% of cases; 70% of patients are younger than 30 years old. This subtype is most often localized and asymptomatic or presents with symptoms of local compression, whereas the plasmacytic type is most often multi-centric and presents with constitutional symptoms.
Our patient’s case was unique owing to her anemia on presentation. We found only 4 cases in the literature reporting anemia, only 2 of which were hyaline-vascular subtypes.1,2 To the best of our knowledge, ours is the first report showing pictures of the pathology.
Unicentric Castlelman disease is a rare discovery on radiographs. No clinical, radiological or cytological features are relevant, images being similar to those seen in lymphoma or thymoma. Diagnosis is exclusively achieved with histological and immunohistochemical findings after resection.
The hyaline-vascular subtype presents germinal centres formed of dysplastic CD21 follicular dendritic cell networks with an expanded mantle zone composed of small CD20 lymphocytes in an onion-skin manner, with extensive interfollicular capillary proliferation and endothelial hyperplasia.3
Studies suggest viral etiologies; no genetic or toxic theory exists. Patients who are HIV-positive are predisposed to plasmacytic disorders.4 Some researchers have suggested the involvement of human herpes virus 8 (HHV-8). Some HHV-8 sequences have been highlighted in the multicentric disease; up to 100% in HIV-positive and 40% in HIV-negative patients.
This herpes virus produces an interleukin-6 homolog, vIL-6, which increases B-cell proliferation (hyperplastic follicles), vascular endothelial growth factor (angiogenesis), Th2-cells (autoimmune reactions) and acute-phase reactants (various B-type symptoms).5 Our patient’s inflammatory anemia may be linked to this vIL-6 mechanism.
In 90%–95% of localized cases, cure consists of surgical resection.1 For unresectable lesions, no progression has been reported after partial excision. Radiotherapy is a possible alternative. The 5-year survival is near 100%.
Castleman disease is a largely ignored disorder with unsolved questions and a wide clinical polymorphism. Unicentric cases may involve not only effects of local mass compression, but also rare systemic symptoms such as anemia, which is the important learning point from this case.
Footnotes
Competing interests: None declared.