Abstract
Background: In a province-wide audit of patients undergoing treatment for rectal cancer in British Columbia in 1996, the 4-year rate of pelvic recurrence for stage 3 rectal cancer was 27%. The management guidelines were changed in 2002 to include adjuvant short-course preoperative radiation and total mesorectal excision surgical techniques. Education workshops were held to implement the protocol change.
Methods: We performed a provincial audit of rectal cancer cases for patients treated in the year after the protocol change, and we compared the pelvic recurrence rates with those from the audit performed in 1996.
Results: During a 12-month period beginning Oct. 1, 2003, a total of 367 patients underwent radical resection of rectal cancer with a curative intent. Preoperative adjuvant radiotherapy was used in 54% of cases (197/367). Median follow-up was 34.5 months, and 91% of patients were followed for at least 2 years. Relative to the 1996 cohort, there was a decreasing trend in 2-year overall pelvic recurrence rates in the 2003/04 cohort (9.6% v. 6.9%) and a significant decrease in recurrence among patients with stage 3 cancers (18.2% v. 9.2%; p = 0.020). Use of adjuvant radiation increased significantly (37% v. 65%; p < 0.001), and negative radial margins were achieved in 87% (319/367) of cases.
Conclusion: The rates of pelvic recurrence were improved after changes in the management guidelines advocating increased use of total mesorectal excision surgery and preoperative radiation. Knowledge translation with an integrated strategy among surgeons and medical and radiation oncologists was successful in improving population outcomes among patients with rectal cancer.
Previously, outcomes for rectal cancer management in British Columbia were reported for 1996.1 In that population-based audit, pelvic recurrence at 4 years was 16% overall and 27% for stage 3 cancer. These data suggested that total mesorectal excision (TME) was not consistently used as the surgical technique for rectal cancer excision. At that time, the BC Cancer Agency’s guidelines for rectal cancer specified that postoperative chemoradiation be given for stage 2 and 3 cancer and that preoperative chemoradiation be given for clinically fixed tumours. Radiotherapy was used in only 37% of patients with rectal cancer.
In a strategy to improve outcomes, the Surgical Oncology Network and the BC Cancer Agency recommended a change in protocol for the management of rectal cancer. The new protocol recommended that resectable (mobile) stage 2 and 3 rectal cancer be treated with adjuvant preoperative short-course radiation (25 Gy given in 5 daily fractions within 1 week before surgery) with TME as the surgical technique; these recommendations were based on excellent outcomes in a Dutch trial.2 To implement this change in protocol, we held TME and rectal management education workshops and included surgeons, pathologists, radiation and medical oncologists.3 In this study, we examined pelvic recurrence and survival and contributing factors after this change in management protocol for rectal cancer in BC.
Methods
Provincial guidelines for adjuvant treatment of rectal cancer in BC were revised in 2002 to recommend preoperative imaging and short-course preoperative radiation for all resectable (mobile) stage 2 and 3 rectal cancer. Preoperative imaging was recommended to assess cancer stage. In most instances, computed tomography was used for preoperative clinical staging because magnetic resonance imaging (MRI) and endorectal ultrasonography were not easily accessible for many patients in the province. We did not record the imaging modality used preoperatively or whether preoperative imaging was performed.
Long-course preoperative radiation (45 Gy given over 4 or more weeks) in combination with 5-fluorouracil (5-FU) chemotherapy was given for tumours that were fixed on clinical examination or for nonpalpable lesions for which imaging showed that the primary tumour or metastatic nodes came sufficiently close to the mesorectal fascia that it was thought to be unlikely that the lesion could be resected with clear margins. The use of short-course treatment requires considerable coordination between surgeons’ offices and radiation therapy departments to ensure that surgery occurs within 7–10 days of radiation. This may necessitate delaying radiation until operating time is available or vice versa. Surgery was arranged 6 to 8 weeks after long-course preoperative chemoradiation. The interval between radiation and surgery was not recorded. Postoperative long-course chemoradiation was recommended for stage 2 and 3 cancer if the patients had not received preoperative treatment. Bolus adjuvant chemotherapy with 5-FU and leucovorin was recommended postoperatively for all patients with stage 2 or 3 tumours.
Between Oct. 1, 2003, and Sept. 30, 2004, the medical records departments for all 42 hospitals in BC were asked to submit operative and pathology reports and discharge summaries for all patients who underwent major resective surgery for rectal cancer. Patients were included if they underwent major resection with curative intent. We excluded patients if they had in situ disease, metastatic disease at presentation or local excision only. Information on adjuvant radiation and chemotherapy was obtained from records at the BC Cancer Agency. Follow-up information was requested from family doctors via a fax of a standardized follow-up data form; the information requested included whether there was recurrent cancer, the site of cancer recurrence and whether the patient was alive. If there was no response to the request for information from the fax, 1 or 2 telephone calls were made to the family doctor to obtain the information. Median follow-up was 48 and 34.5 months for the 1996 and 2003/04 cohorts, respectively. In the latter cohort, 91% of patients were followed for at least 2 years.
We grouped patients by age, sex, tumour location (upper [11–15 cm], mid [6–10 cm] or distal [1–5 cm] rectum) according to the distance from the anus, cancer stage, surgery type (sphincter-preserving [low anterior resection, Hartmann] v. permanent colostomy), adjuvant radiation and chemotherapy type, and involvement of the circumferential resection margin (CRM). A negative margin was recorded if the distance from the tumour to the circumferential margin was more than 1 mm. Owing to the high rate of pelvic recurrence among those with stage 3 cancer in 1996, we performed a subgroup analysis comparing stage 3 cancers in the 1996 and 2003/04 cohorts.
We used the Student t test to compare the 2 cohorts with respect to age at diagnosis, and we used the χ2 test to compare all other patient characteristics. Nonparametric estimates of the survivor functions for pelvic recurrence were computed using the Kaplan–Meier method, and the 2 cohorts were compared using the log-rank test. We used the Cox proportional hazards model to compare the cohorts after adjusting for differences in patient characteristics and to identify factors predictive of pelvic recurrence. Univariate calculated rates of recurrence were not given because that would result in data tables of recurrence rates with confidence intervals for too many multiple factors (18 for overall stage recurrence and 12 for stage 3 recurrence). We excluded patients with unknown values from the analyses.
This study received approval from the University of British Columbia and the BC Cancer Agency’s Research Ethics Board.
Results
In 1996, a total of 495 patients were identified from the BC Cancer Registry as having rectal or rectosigmoid adenocarcinoma. Of these, we excluded 212 because of incomplete data, in situ or metastatic cancer, unknown distance from the anal verge or because the surgical procedure was polypectomy or local excision. In the 2003/04 cohort, 481 patients were identified from hospital records as having undergone radical surgical treatment for rectal cancer. Of these, we excluded 114 patients because of in situ disease, local excision or palliative diverting stoma. Thus, we included 283 patients from the 1996 cohort and 367 from the 2003/04.
Table 1 presents the demographic and tumour characteristics of the 1996 and 2003/04 cohorts. The cohorts were not different with respect to age, sex ratio or restorative resection surgery. Tumour distance from the anal verge was different between the cohorts; there was a higher proportion of distal rectal cancers in the 2003/04 cohort (p < 0.001). There was a trend toward more advanced stage disease in the 2003/04 cohort.
Characteristics of the 1996 and 2003/04 cohorts
In the 2003/04 cohort, 151 of 367 patients (41%) received short-course preoperative radiation, 46 of 367 patients (13%) received preoperative long-course radiation and chemotherapy, 40 of 367 patients (11%) received postoperative radiation and chemotherapy, and 130 of 367 patients (35%) did not receive radiation. The use of adjuvant radiation was significantly higher in the 2003/04 cohort (65% v. 37%; p < 0.001). More patients received preoperative radiation in 2003/04, with a ratio of preoperative to postoperative adjuvant radiation of 3.5% to 33.2% in 1996 and a ratio of 53.5% to 10.8% in 2003/04.
Long-course preoperative radiation was used in 25 patients with stage T3/T4 cancer in 2003/04; there were 3 pelvic recurrences in this group. Short-course preoperative radiation was used in 94 patients with T3/T4 cancer in 2003/04; there were 2 pelvic recurrences in this group. Because the number of patients with pelvic recurrence was small in these groups, we did not include type of preoperative radiation in the multivariate analysis. In addition, 57 patients with T0/T1/T2 cancer in the 2003/04 cohort received short-course preoperative radiation.
In 2003/04, the negative CRM rate was 86.9%. Unknown CRM status was significantly more common in 1996 than in 2003/04 (54.0% v 2.9%; p < 0.001). Lymph node counts increased from a mean of 6.3 (standard deviation [SD] 5.0) in 1996 to 11.7 (SD 6.7) in 2003/04 (p < 0.001).
The number of surgeons was 110 and 106 for the 1996 and 2003/04 cohorts, respectively. The median number of procedures per surgeon was 2 (range 1–11) in 1996 and 2.5 (range 1–24) in 2003/04. Most surgeons performed less than 5 operations per year (85% in 1996 and 76% in 2003/04). The median number of cases per hospital was 7 (range 1–24) in 1996 and 11 (range 1–27) in 2003/04. The mean number of surgeons who performed rectal cancer surgery per hospital was 3 (range 1–8) in 1996 and 4 (range 1–9) in 2003/04. We did not analyze the effect of surgeon or hospital volume on outcomes because of these small numbers.
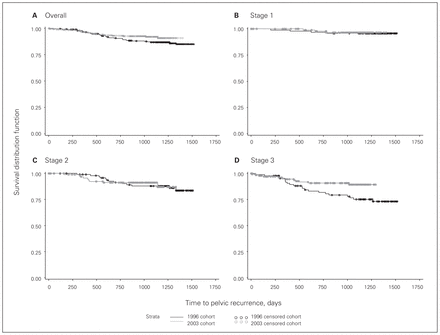
There was a trend toward a lower 2-year overall pelvic recurrence rate in the 2003/04 cohort (Fig. 1). The overall 2-year pelvic recurrence rate was 9.6% in 1996, compared with 6.9% in 2003/04 (p = 0.11). Although there was no difference for stages 1 and 2, patients with stage 3 cancer had a significantly lower rate of pelvic recurrence (stage 1, 3.2% v. 2.3%; stage 2, 8.7% v. 8.8%; stage 3, 18.2% v. 9.2%; p = 0.020).
Pelvic recurrence among (A) all patients and among those with (B) stage 1, (C) stage 2 or (D) stage 3 cancer.
Using a Cox regression model, we found that pelvic recurrence for the entire cohort was significantly influenced by stage, adjuvant radiation treatment and CRM status. Distance from the anal verge, type of surgical resection, sex and age did not significantly affect pelvic recurrence in the entire cohort (Table 2). We also performed a subgroup analysis of patients with stage 3 cancer. The demographic characteristics of patients with stage 3 cancer are shown in Table 3. Similar to the entire cohort, there were more distal-third cancers in the 2003/04 cohort than in the 1996 cohort (37.8% v. 22.4%). Although the use of adjuvant radiation was not different between the stage 3 subgroups (77.7% v. 69.7%), there was increased use of preoperative radiation in the latter cohort (61.4% v. 4.4%; p < 0.001). A multivariate Cox regression analysis of stage 3 patients showed a significantly higher risk of pelvic recurrence for distal-third rectal location compared with the upper- and middle-third location and with anterior or Hartmann resection compared with abdominoperineal resection (Table 4).
Factors influencing pelvic recurrence rates
Characteristics of patients with stage 3 cancer
Factors affecting pelvic recurrence in patients with stage 3 cancer
The 2-year overall survival rates for the 1996 and 2003/04 cohorts were 88% and 85%, respectively, and the 2-year disease-specific survival rates were 94% and 90%, respectively. Although overall survival was not different between cohorts (p = 0.14), disease-specific survival was higher for the 1996 cohort than the 2003/04 cohort (p = 0.043; Fig. 2). In a multivariate analysis, stage was the most significant factor affecting disease-specific survival (p < 0.001).
Probability of (A) overall and (B) disease-specific survival.
Discussion
We found decreased pelvic recurrence among patients with stage 3 cancer in a province-wide audit of patients who underwent rectal cancer surgery after changes in the provincial guidelines for rectal cancer management were instituted. There was increased use of adjuvant radiation overall as well as increased use of preoperative radiation in keeping with management guidelines. Surgical CRM status reporting improved significantly, and CRM negative status was in keeping with TME as the surgical technique for rectal cancer excision.
Similar to the Dutch,2 Swedish4 and Norwegian5 initiatives, the BC Cancer Agency and the Surgical Oncology Network changed their guidelines for rectal cancer management to recommend the use of preoperative short-course radiation and TME surgery. The resulting decrease in pelvic recurrence in BC is similar to findings from those national studies after their management guidelines were changed. In particular, the 2-year pelvic recurrence rate in the Dutch national trial was 5.3% overall and 9.9% for patients with stage 3 cancer.2 In our study, the 2-year pelvic recurrence rates for the 2003/04 cohort was similar (6.9% overall, 9.2% for stage 3 cancers). These data show similar results in a population-based setting to the outcomes of clinical trials in Europe and demonstrate the importance of periodic evaluation of outcomes and the promotion of cancer management guidelines.
Changes in the management guidelines were implemented via TME and rectal management education workshops offered to surgeons, pathologists and radiation and medical oncologists in BC.3 Although attendance was voluntary, we estimate that 80% of general surgeons in BC attended the courses and presume that many of the remaining surgeons were already trained in TME or were no longer caring for patients with rectal cancer. Our negative CRM rate of 86.9% is in keeping with the 85% negative rate reported in the Dutch TME trial, in which all participating surgeons were trained in TME techniques.6,7 We were pleased that pathologists improved their reporting of CRM: in 1996, CRM status was not reported in 54% of cases, whereas it was not reported in only 2.9% of cases in the 2003/04 cohort. We could not compare CRMs between cohorts because of the low rate of CRM assessment in 1996.
Although the use of sphincter-preserving surgical techniques was not different between cohorts, there were more distal rectal cancers (< 5 cm from anal verge) in the 2003/04 cohort. This suggests that patients eligible for sphincter preservation were more likely to receive this treatment in the latter cohort. Increased sphincter-preserving resection has been used as an indicator of adoption of the TME technique.8
There are 2 aspects of distal-third rectal cancer location that have no satisfactory explanation. First, there were more distal-third cancers in the latter cohort. This finding could have resulted from the relatively small sample size or the exclusion of patients owing to incomplete data or both. Second, the subgroup analysis of stage 3 cancers showed significantly higher pelvic recurrence among patients with distal-third tumours treated with sphincter-preserving surgical resection. Data from the Dutch trial2 showed similarly higher positive CRMs for distal-third rectal locations. However, their data indicated higher perforation and local recurrence rates using abdominoperineal resection, which contradicts our findings. We agree that particular care should be taken by surgeons to achieve wide radial margins for cancers involving the levators.9
The use of adjuvant preoperative short course radiation in the 2003/04 cohort was a significant factor affecting pelvic recurrence. Nearly twice the number of patients in the 2003/04 cohort received adjuvant radiotherapy compared with the 1996 cohort, and the emphasis had changed from predominantly postoperative to preoperative treatment. The change from postoperative to preoperative radiotherapy in stage 3 cancers appears to have contributed to the lower rate of pelvic recurrence rate in stage 3 patients in the latter cohort. Potential overtreatment of T1/T2 lesions with preoperative radiation is of concern, especially if preoperative imaging is not routinely performed. Because we have reported pathologic stage rather than preoperative clinical stage, downstaging could explain why some T1/T2 lesions received preoperative radiation. We did not collect information about other complications of preoperative radiation such as anastomotic leak, use of defunctioning stoma and rectal and urogenital dysfunction. A major component of the rectal management education workshops was the promotion of the provincial guidelines and the improvement of the multimodality care of these patients. The increased use of radiotherapy suggests that this knowledge-translation initiative was successful.
Most of the patients in the 2003/04 cohort received preoperative short-course radiotherapy. On the basis of the good outcomes, we continue to favour the use of preoperative adjuvant radiation over postoperative treatment, which is in keeping with the results of the German trial.10 Although preoperative short-course radiation is as effective for cancer control as preoperative long-course chemoradiation is for mobile cancers,11 we currently recommend long-course chemoradiation to achieve maximum downstaging in cases of clinical fixation, tumours that are abutting the mesorectal fascia on preoperative imaging and those located close to the sphincter. Owing to the inconsistency of preoperative imaging, there is uncertainty about clinical stage and recommendation for preoperative adjuvant radiation was not predicated on MRI criteria. Preoperative MRI is recommended for T3/T4 lesions because, at present, MRI provides the best assessment of whether the predicted radial margin is involved. At this time, we do not review all cases in a multidisciplinary conference. Difficult cases are reviewed at a multidisciplinary conference. Locally advanced disease was not coded in either cohort. Long-course preoperative radiation was used in 25 patients with T3/T4 cancer in 2003/04, with 3 pelvic recurrences. Short-course preoperative radiation was used in 93 patients with T3/T4 cancer in 2003/04, with pelvic recurrence in 2. Because the number of patients with pelvic recurrence was small in these groups, we did not include this variable in the multivariate analysis. Reasons for not receiving adjuvant radiation were not recorded but likely included patient refusal and comorbidity. We did not record comorbidity in our data collection.
Overall survival was not different between cohorts, which is in keeping with most other studies of adjuvant pelvic radiation for rectal cancer.4,5,7 However, disease-specific survival was significantly lower in the 2003/04 cohort. Because cancer stage was the most significant factor affecting disease-specific survival, it is likely that the higher percentage of stage 3 cancers in the 2003/04 cohort than in the 1996 cohort (40.3% v. 31.4%, respectively) accounts for the lower disease-specific survival in the later cohort. Age and radiation treatment also significantly affected disease-specific survival. Differences in the disease-specific survival curves seem to occur early. We do not have data that explains this observation, but the possibilities include aggressive differences within stage 3 disease and miscoding of cancer-related death.
We did not perform analysis of the effect of surgeon or hospital volume on outcomes because of the small numbers and wide frequency distribution of cases. To date, we have not instituted any policy to define criteria for performing rectal cancer surgery. Surgeons refer difficult cases to higher volume centres based on their judgment of their technical and hospital capabilities. Although outcomes intuitively might improve by formalizing rectal cancer surgery centres and requirements for referring patients to such centres, there are significant political, economic and patient-preference barriers to creating such a formal system without definitive evidence of further improvement in outcomes.
Although we performed a population study involving many surgeons and oncologists, the numbers are small. Further, the retrospective nature of the study introduces potential for bias, particularly with respect to more deletions in 1996 owing to incomplete follow-up records.
Conclusion
Education courses about TME surgery and preoperative short-course radiation have resulted in decreased pelvic recurrences in BC. Although this strategy has been reported in Europe, to our knowledge this is the first report of an education strategy to improve rectal cancer outcomes for a population-based cohort in North America. We used multi-disciplinary TME education and rectal cancer management workshops to initiate this change, and we suggest that knowledge translation is an effective strategy to implement evidence-based guidelines designed to improve cancer outcomes in a population setting. There is little research, outside of teaching technical skills, about continuing medical education specifically for surgeons and what exactly works. Our project combined a number of modalities: conferences, the inclusion of opinion leaders, reinforcement through retesting, and presentations about outcomes at serial meetings, to achieve its goals.12–14
Acknowledgements
The authors thank Chrystal Palaty for data retrieval and Yasmin Miller for organizational support. This study was funded by the British Columbia Surgical Oncology Network as a quality-improvement initiative.
Footnotes
Competing interests: None declared.
Contributors: Drs. Phang and Cheifetz designed the study. Drs. Phang, MacFarlane and Hay and Ms. McGahan acquired the data, which Drs. Phang, Brown, Raval and Hay and Ms. McGahan analyzed. Drs. Phang, McGregor and Raval and Ms. McGahan wrote the article. All authors reviewed the article and approved its publication.
- Accepted November 26, 2009.