Abstract
Background: Cardiac retransplantation remains the most viable option for patients with allograft heart failure; however, careful patient selection is paramount considering limited allograft resources. We analyzed clinical outcomes following retransplantation in an academic, tertiary care institution.
Methods: Between 1981 and 2011, 593 heart transplantations, including 22 retransplantations were performed at our institution. We analyzed the preoperative demographic characteristics, cause of allograft loss, short- and long-term surgical outcomes and cause of death among patients who had cardiac retransplantations.
Results: Twenty-two patients underwent retransplantation: 10 for graft vascular disease, 7 for acute rejection and 5 for primary graft failure. Mean age at retransplantation was 43 (standard deviation [SD] 15) years; 6 patients were women. Thirteen patients were critically ill preoperatively, requiring inotropes and/or mechanical support. The median interval between primary and retransplantation was 2.2 (range 0–16) years. Thirty-day mortality was 31.8%, and conditional (> 30 d) 1-, 5- and 10-year survival after retransplantation were 93%, 79% and 59%, respectively. A diagnosis of allograft vasculopathy (p = 0.008) and an interval between primary and retransplantation greater than 1 year (p = 0.016) had a significantly favourable impact on 30-day mortality. The median and mean survival after retransplantation were 3.3 and 5 (SD 6, range 0–18) years, respectively; graft vascular disease and multiorgan failure were the most common causes of death.
Conclusion: Long-term outcomes for primary and retransplantation are similar if patients survive the 30-day postoperative period. Retransplantation within 1 year of the primary transplantation resulted in a high perioperative mortality and thus may be a contraindication to retransplantation.
Survival after cardiac transplantation has improved substantially over recent decades with improved organ preservation, development of better tolerated immunosuppressive medications and careful patient selection. Multidisciplinary transplantation programs have become better established, providing more comprehensive and long-term follow-up resulting in improved late outcomes and patient survival. As a consequence, an increasing number of patients present with late allograft failure requiring retransplantation, accounting for 2.4% of adult and 8% of pediatric cardiac transplantation in the 2010 International Society for Heart and Lung Transplantation registry.1,2 Implantation of ventricular assist devices as destination therapy has been another option for end-stage heart failure; however, midterm clinical outcomes have been disappointing, and widespread acceptance of this therapy has been limited.3,4 Cardiac retransplantation remains the most promising option for most of these patients with failing allografts, although there is controversy surrounding the appropriateness of retransplantation because of limited organ availability and previously reported poor results.5–11
We sought to evaluate our 30-year single-centre experience with cardiac retransplantation to define early and late patient outcomes and to better characterize optimal patient selection criteria.
Methods
Participants
We retrospectively reviewed the cases of orthotopic heart transplantation for end-stage heart failure that took place at the University of Western Ontario in London, Ontario, between April 1981 and January 2011. Patients who received retransplantation were thoroughly evaluated and selected by a multidisciplinary medical and surgical committee. Exclusion criteria were similar to those for primary transplantation, including fixed pulmonary hypertension, systemic illness including malignancy, peripheral vascular disease, irreversible end-organ damage and poor compliance. Panel reactive antibodies were performed in all patients preoperatively and were defined as positive if they had 50% or higher reactivity. Computed tomography of the chest was also performed to evaluate the extent of adhesions between the heart and sternum. For organ preservation, University of Wisconsin and Celsior solutions were used according to the era in which the transplantation occurred. Implantation techniques included either biatrial or bicaval anastomosis, depending on surgeon preference. We investigated short-term outcomes, including 30-day mortality, perioperative morbidity, stay in the intensive care unit and total length of stay in hospital, and long-term outcomes, such as survival and causes of death after retransplantation. We performed a univariate analysis to determine risk factors associated with 30-day operative mortality among patients undergoing retransplantation. Conditional survival was defined as survival beyond the first 30 postoperative days.
Approval for data collection for this study was granted by the Research Ethics Board of the University of Western Ontario, which waived the requirement for informed consent from individual patients.
Immunosuppressive regimen and follow-up
Postoperative immunosuppression consisted of a triple maintenance therapy based on cyclosporine or tacrolimus in combination with azathioprine or mycophenolate and steroids. Azathioprine and cyclosporine were preferred in the 1990s, and mycophenolate mofetil and tacrolimus were preferred after 2000. Antithymocyte globulin was given after retransplantation when it was not used in the previous transplantation. All patients were followed up in our transplant outpatient clinic with routine echocardiography, endomyocardial biopsy and right and left heart catheterizations performed at protocalized intervals.
Statistical analysis
Continuous variables are presented as means and standard deviations (SD), with comparisons performed using 2-tailed t tests. Categorical variables are presented as a proportion and were compared using χ2 analysis or the Fisher exact test. We performed a Cox proportional hazard analysis to determine the factors associated with mortality within 30 days after retransplantation. Actuarial survival after retransplantation was estimated with the Kaplan–Meier method and analyzed using the log rank test. Furthermore, conditional postretransplantation survival, defined as survival longer than 30 days postoperatively, was also calculated. StatView Version 5.0 (SAS Institute Inc.) was used to perform statistical analyses. We considered results to be significant at p < 0.05, 2-sided.
Results
Participants
A total of 593 orthotopic heart transplantations for end-stage heart failure took place during our study period. Of these, 22 (3.7%) were cardiac retransplantations. Preoperative patient profiles are shown in Table 1. Most recipients were men. The mean age at retransplantation was 43 (SD 15) years. The mean interval between primary transplantation and retransplantation 5.1 (SD 5.4, median 2.2, range 0–16) years. Indications for surgery were graft vascular disease in 10 (45.5%) patients, acute rejection in 7 (31.8%) and primary graft failure in 5 (22.7%). The preoperative general condition, as defined by Canadian Society of Transplantation criteria, was critical for most patients: 13 (59.1%) patients were in status 3 or 4 (i.e., requiring inotropic support/ventricular assist devices or dependent on mechanical ventilation/circulatory support in the intensive care unit, respectively) immediately before retransplantation. All patients whose retransplantations took place in the earlier half of the study period (1984–1994, n = 12) were either in status 3 or 4, whereas 9 of 10 patients whose retransplantations occurred after 1994 were in status 1 (i.e., stable at home). The mean donor age was 31.2 (SD 14.3) years, and the mean total ischemic time was 245 (SD 100) minutes.
Demographic characteristics of 22 patients who underwent cardiac retransplantation
Short- and long-term outcomes after cardiac retransplantation
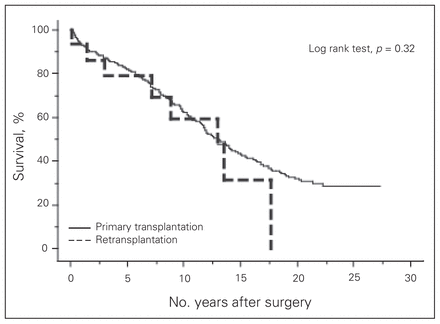
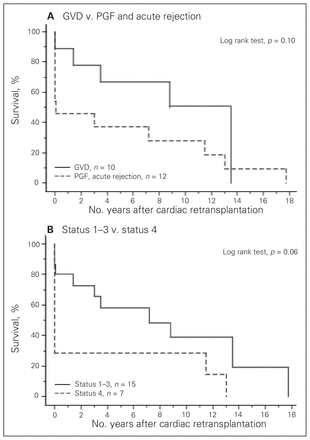
Short-term outcomes and causes of death after cardiac retransplantation are described in Tables 2 and 3. Overall 30-day mortality was 31.8%, whereas that for patients with graft vascular disease was 10% (1 of 10 patients) and that for patients with acute rejection or primary graft failure was 50% (6 of 12 patients). Multisystem organ failure was the most prominent cause of perioperative death. Four of the 5 patients who died of multisystem organ failure received retransplantation for either acute graft rejection or primary graft failure a very short time after the primary transplantation surgery. In 2 of these 4 patients, multisystem organ failure was triggered in the primary transplantation for primary graft failure 1 day before the retransplantation, and both patients were unable to recover from their critical conditions even after they received well-functioning hearts. One patient went into multisystem organ failure as a consequence of acute allograft rejection from a primary heart transplantation that took place 14 days prior to retransplantation. In the fourth patient, acute rejection became chronic rejection with a long duration of graft malfunction resulting in irreversible multisystem organ failure. These 4 cases of multisystem organ failure occurred earlier in our transplantation experience. Most patients who received retransplantation for graft vascular disease responded well and had a good long-term prognosis. Figure 1 presents the conditional survival of the patients after retransplantation (n = 15), with conditional survival after primary cardiac transplantation (n = 448) for comparison. The conditional 1-, 5- and 10-year survival for retransplantation versus primary transplantation survivors at our institution were 93.3%, 79.0% and 59.2% versus 93.0%, 82.0% and 62.7%, respectively, with no significant difference between the groups (p = 0.32). The mean and median durations of graft survival after retransplantation were 5.0 and 3.3 (SD 5.7, range 0–17.7) years, respectively. Figure 2 represents the comparison of Kaplan–Meier curves between patients with and without graft vascular disease (Fig. 2A) and between patients with a preoperative status of 1–3 and those with a preoperative status of 4 (Fig. 2B). Although neither curve reached statistical significance, both demonstrated strong tendencies toward improved survival in that patients with graft vascular disease and patients with a preoperative status of 1–3 had better prognoses than those with a different condition or with a preoperative status of 4.
Conditional (> 30 day) survival for primary transplantation and retransplantation from our 30-year experience (p = 0.32).
Kaplan–Meier curves comparing long-term survival between (A) patients with graft vascular disease (GVD) versus non-GVD (p = 0.10), and between (B) patients with a preoperative status of 1–3 versus status 4 (p = 0.06). PGF = primary graft failure.
Early and late outcomes of 22 patients who underwent cardiac retransplantation
Causes of death among 22 patients who underwent cardiac retransplantation
Risk factors affecting survival after cardiac retransplantation
Risk factors affecting survival after retransplantation are shown in Table 4. An interval between the primary and retransplantation of 1 year or less and the indication for retransplantation were significant risk factors affecting survival. Patients who had retransplantation for graft vascular disease and those who had an interval longer than 1 year after the initial transplantation lived longer than patients with other conditions or with shorter intervals between the initial and retransplantation (Table 5).
Risk factors with a negative impact on survival after retransplantation
Impact of time interval between initial and retransplantations on survival outcomes
Discussion
The appropriateness of cardiac retransplantation has been a matter of discussion since the 1980s and remains controversial owing to limited organ and resource availability and perceived inferiority in its long-term outcome compared with primary transplantation. A limited number of institutions have reported their long-term clinical results with cardiac retransplantation, and most outcomes have not been favourable compared with outcomes of primary transplantation. They have reported 1-year survival between 45.5% and 86.6% and 5-year survival between 30% and 73%.5–13 Most reports suggest caution about retransplantation because its outcomes are inferior to those of primary transplantation, and conclude that retransplantation should thus be considered only for select patients. On the other hand, Atluri and colleagues12 found that meticulous perioperative care improved the results of retransplantation and concluded that it was an efficacious procedure with potentially wider application. A recent report on 23 patients who had retransplantations over a 25-year period at an experienced heart transplant centre suggested improved late survival results when repeat transplantation was performed for graft vascular disease or chronic graft failure.14 When examining their entire transplant cohort, the authors were also able to demonstrate similar early and late survival results between primary and retransplantation patients.14 The present series involved a very sick cohort of patients in critical condition following their primary transplantation who underwent retransplantation, which likely contributed to the high incidence of multisystem organ failure and subsequent 30-day mortality. Most of our patients with poor early outcomes were treated early in our 30-year experience. The patients who survived 30 days after retransplantation experienced relatively good long-term outcomes that were comparable to those for primary transplantation, which is in keeping with recently published series.12,14 We believe our report is the largest published series on retransplantation in Canada. With experience, we now recognize that retransplantation is most successful in patients who have graft vascular disease or chronic graft failure, an interval of more than 1 year between the initial and retransplantation and relatively stable conditions preoperatively.
Acute graft failure following primary transplantation presents a difficult clinical dilemma. Left ventricular assist devices (LVADs) have been widely adopted by most transplantation programs to stabilize decompensated heart failure patients awaiting primary transplantation; recent publications have suggested that long-term outcomes for patients with preoperative LVADs were comparable to results for those without.15–17 Patients in our series had intra-aortic balloon pumps and extracorporeal membrane oxygenation available, but no other LVADs or mechanical assist devices were used until recently.
The indication for retransplantation was another key issue affecting patient outcomes. The analysis from our series revealed that retransplantation for graft vascular disease significantly improved 30-day mortality and late survival compared with retransplantation for other conditions. These findings are supported by others, who report similarly good outcomes in patients who had retransplantation for graft vascular disease;10,11,18,19 however, patients with acute graft rejection or primary graft failure had significantly inferior outcomes.10,11,18,20 We therefore conclude that allograft failure for acute graft rejection or primary graft failure should be medically managed and the patients possibly excluded from consideration for retransplantation.
The interval between primary and retransplantation was another significant factor affecting the outcome in our series. This has also been reported in other studies where shorter intervals between operations significantly increased adverse outcomes.11,18 Because of this, most authors generally advise against retransplantation less than 1 month after primary transplantation because of clearly inferior outcomes.
Limitations
The strength of our study is that, to our knowledge, it is the largest series of cardiac retransplantation in Canada. Limitations include the retrospective case series design that could have been subject to temporal biases. Nonetheless, our data are similar to other reported series with respect to quality and patient size and, considering the rarity of this clinical scenario, represent the best available information to guide clinical decision-making.
Conclusion
Cardiac retransplantation can provide satisfactory early and long-term outcomes if patients are carefully selected. Transplant patients who have graft vascular disease and have survived longer than 1-year beyond their primary transplantation have the best clinical outcomes, similar to those of primary transplantation. Conversely, retransplantation within 1 year was associated with a very high mortality, and a short interval between the transplant surgeries may be a relative contraindication to retransplantation. Indication for retransplantation, particularly for acute rejection or primary graft failure, appears to be a major determinant of inferior perioperative outcomes. Careful patient selection is paramount when considering heart retransplantation.
Footnotes
Competing interests: None declared for A. Saito, R.J. Novick, F.N. McKenzie, M. Quantz, P. Pflugfelder, G. Fisher and M.W.A. Chu. B. Kiaii declares having consulted for Medtronic of Canada Ltd. and St. Jude Medical Canada Inc., and having received honoraria from them.
Contributors: A. Saito, R.J. Novick, B. Kiaii, P. Pflugfelder and M.W.A. Chu designed the study. A. Saito, R.J. Novick, F.N. McKenzie, M. Quantz, P. Pflugfelder and G. Fisher acquired the data. A. Saito, R.J. Novick, P. Pflugfelder and M.W.A. Chu analyzed the data. A. Saito, R.J. Novick, P. Pflugfelder and M.W.A. Chu wrote the article. A. Saito, B. Kiaii, F.N. McKenzie, M. Quantz, P. Pflugfelder, G. Fisher and M.W.A. Chu reviewed the article. All authors approved its publication.
- Accepted August 16, 2011.