Abstract
Background: The consolidation of acute care surgery (ACS) services at 3 of 6 hospitals in a Canadian health region sought to alleviate a relative shortage of surgeons able to take emergency call. We examined how this affected patient access and outcomes.
Methods: Using the generalized linear model and statistical process control, we analyzed ACS-related episodes that occurred between 39 months prior to and 17 months after the model’s implementation (n = 14 713).
Results: Time to surgery increased after the consolidation. Wait times increased primarily for patients presenting at nonreferral hospitals who were likely to require transfer to a referral hospital. Although ACS teams enabled referral hospitals to handle a much higher volume of patients without increasing within-hospital wait times, overall system wait times were lengthened by the growing frequency of patient transfers. Wait times for inpatient admission were difficult to interpret because there was a trend toward admitting patients directly to the ACS service, bypassing the emergency department (ED). For patients who did go through the ED, wait times for inpatient admission increased after the consolidation; however, this trend was cancelled out by the apparently zero waits of patients who bypassed the ED. Regionalization showed no impact on length of stay, readmissions, mortality or complications.
Conclusion: Consolidation enabled the region to ensure adequate surgical coverage without harming patients. The need to transfer patients who presented at nonreferral hospitals led to longer waits.
Access to emergency or urgent surgery has become a serious concern across North America and beyond.1,2 With the evolution of surgical subspecialties there has been a relative decline in the availability of surgeons who provide emergency care, particularly after hours.3–5 Many health care organizations and systems have sought to cope with the problem by consolidating surgical services. There is growing international interest in the acute care surgery (ACS) model, in which designated resources, including the time of designated surgeons, are set aside for emergency and/or urgent surgery. The model may be implemented within a hospital, among hospitals (regionalization), or both.
Past research has suggested that within-hospital ACS consolidation can reduce wait times for emergency surgery, and may sometimes improve patient outcomes.6–9 However, the literature is dominated by uncontrolled pre–post studies, which cannot rule out secular trends and other confounders, such as adoption of new surgical practices, as explanations for the observed results. Very few studies have evaluated ACS consolidation on a regional level involving multiple institutions. One study found that mortality and length of stay (LOS) decreased over time within a regionalized ACS service, but it did not compare these rates with those observed before regionalization.10 Another study found no significant change in mortality when high-acuity surgery (including many procedures outside the scope of emergency general surgery) was regionalized; there was an ongoing decline in LOS, but this decline had begun before regionalization.11 To our knowledge, no study has examined wait times at the pan-regional level, taking into account how transfers affect the patient journey. As the ACS model becomes increasingly widespread, there is an urgent need for further evidence on the impacts of ACS consolidation, especially regional consolidation.
The Winnipeg Regional Health Authority (WRHA), an urban health region in Western Canada (catchment population 1.2 million), implemented the ACS model to stabilize and sustain call schedules. The region had been unable to fill the gaps through recruitment because there was neither the volume of work nor the resources to support new recruits. This 6-hospital system consolidated emergency general surgery at 3 referral sites, each of which now includes a designated ACS team. A clinical lead or service chief (funded position) is responsible for addressing administrative needs and maintaining call schedules. One surgeon covers the day shift (7:30–16:30) from Monday to Sunday, providing continuity throughout the week. The night shift (16:30–7:30, home-call) rotates among participating surgeons on a daily basis. Remuneration is a blended model of a day and night stipend and fee-for-service. A hospitalist was added to the ACS team at the nonteaching site; hospitalists and/or physician assistants play a smaller role at the teaching sites. The referral hospitals have added daytime ACS slates, but not dedicated emergency operating rooms (ORs). To offset the increased volume of ACS patients at the referral hospitals, both elective general surgery and orthopedic surgery cases have been relocated to the nonreferral hospitals.
One hospital became a referral centre in April 2008, another in December 2008. (A third offered ACS throughout the study period but did not invite additional referrals.) The consolidation succeeded in filling call schedules and ensuring reliable access to an on-call surgeon. This study sought to determine the impact of consolidation on patient access and outcomes.
Methods
Our analyses of administrative data included all adult patients (aged 20 years and older) with an ACS-related in-patient stay at a WRHA hospital between 2005 and 2009. The University of Manitoba Health Research Ethics Board approved our use of this deidentified data for research purposes. We defined “ACS-related” as an emergent admission to the general surgery service, where the most responsible diagnosis was gastrointestinal (GI)-related (ICD-10 K codes [diseases of the GI system] or R10 codes [abdominal pain]). This criterion was used because the most common acute surgical conditions are GI-related (e.g., appendicitis, cholecystitis), whereas the most common types of emergency general surgery outside the scope of the ACS consolidation (trauma and cancer-related surgery) are not. We defined an “episode of care” as an acute care admission with an eligible diagnosis and emergency department (ED) visit that occurred within 24 hours (or, if a patient transfer was reported, within 72 h) of another admission or visit. Based on these criteria, we arrived at a sample of 14 735 episodes of care. In the interest of statistical independence of observations, we then excluded episodes where the patient had a recent prior episode. For tests of system responsiveness (time to surgery, time to inpatient admission) and complications, we considered a recent episode to have occurred in the previous 72 hours; for other tests of patient management, we considered a recent episode to have occurred in the previous 30 days.
The dependent variables included time to surgery (time from first presentation at a WRHA hospital to first surgery), time to inpatient admission (time from first presentation at a WRHA hospital to first inpatient admission), LOS (total time from first inpatient admission to last in-patient discharge), readmission (all-cause readmission to any WRHA hospital within 30 d of being discharged alive), death (in hospital or within 30 d of discharge) and complications (presence of 1 or more complications in surgical patients; the data source included generalized complications, such as infection, hemorrhage and iatrogenic injury, but not disease-specific issues, such as ruptured appendix).
The intervention period began on Apr. 1, 2008, with the creation of the first referral site. Given the stepped nature of implementation, we considered dividing the intervention period into 2 phases; however, phase 2 and its interaction with linear time never reached significance in any model and were therefore removed. Covariates included month of last hospital discharge, sex, age, diagnostic category (appendicitis, cholecystitis, intestinal obstruction, pancreatitis, diverticulitis, other), operative status (undergoing an ACS-related procedure within 7 d of the start of the episode; the list of eligible procedures was determined by a surgeon who was blind to all other data), non-WRHA facility (transfer to or from out-of-region facility during the episode) and referral hospital (presenting at a hospital that was or became a referral centre). The inclusion and exclusion criteria and related sample sizes for each analysis are provided in Table 1, and characteristics of the sample are summarized in Table 2.
Inclusion and exclusion criteria
Characteristics of the study sample
Statistical analysis
Analytic methods included statistical process control and regression modelling. Statistical process control (SPC) involves plotting the data on a control chart to examine the timing and magnitude of any changes.12,13 Results are tested for significance according to rules that include 1 data point outside the upper and lower control limits, 6 consecutive data points ascending or descending and 9 consecutive data points above or below the mean. On the control charts, the solid line represents the mean and the dotted lines represent the upper and lower control limits. We calculated these values based on the preintervention period.
We used multiple linear regression for continuous (log-transformed) variables and logistic regression for binary variables. Before choosing this method, we used the Durbin–Watson test to check for autocorrelation of errors, using data at the levels of both individual cases and monthly aggregates.14 These tests did not show significant results (the Durbin–Watson statistic approached 2), indicating that it was unnecessary to use a procedure, such as ARIMA, to control for autocorrelation.
Results
Implementation of ACS
Between 2007 and 2009, ACS all but stopped at the 3 non-referral centres, while the proportion of ACS episodes handled at the 2 new referral centres increased by 71.5%. The overall volume of general surgery patients rose permanently at 1 referral centre and temporarily at the other (it should be noted that the consolidation also redistributed non–general surgery patients). A 4.5-fold increase was observed in patient transfers among WRHA hospitals for surgical consultation or treatment. Ambulance data suggested that this increase was somewhat, but not fully offset by a decrease in the number of there-and-back transfers for tests, such as computed tomography (see the Appendix, Figs. S1 and S2, available at cma.ca/cjs).
Time to surgery
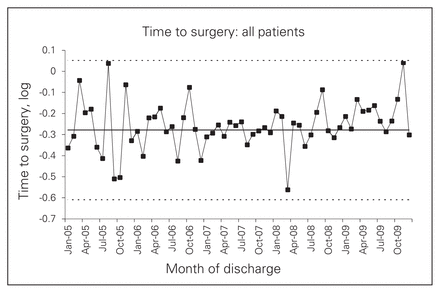
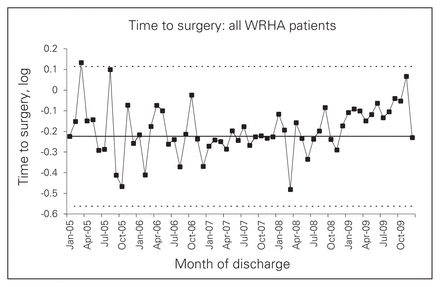
Our SPC analysis suggested some increase in wait times after the intervention, although this trend did not reach significance (Fig. 1). However, Figure 2 shows that when the analysis was repeated for patients within the WRHA, the finding clearly reached significance (more than 9 points above the preintervention mean, with the rise beginning around December 2008). This finding was explained by the observation that the vast majority of non-WRHA patients presented to 1 of the 3 ACS referral hospitals directly, whereas about one-third of WRHA patients presented to a non-ACS site (both before and after the intervention). Further analysis confirmed that, whereas wait times stayed fairly constant at referral hospitals, they rose sharply at feeder hospitals after the intervention began (see the Appendix, Figs. S3 and S4). This rise appeared to reflect the increasing proportion of patients who were transferred (from 18.8% to 70.6% of surgical patients presenting at nonreferral hospitals), not an increase in the length of time associated with a transfer (which did not change over the study period). Supplementary analyses indicated that transferred patients waited a median of 5.5 hours longer than nontransferred patients; depending on whether the patient travelled by ambulance, 2–3 of these hours could be accounted for by the transfer process. The remaining time appeared to reflect pre- and posttransfer delays, such as waiting for access to a bed or OR suite. The increased wait did not seem to occur as a result of arriving at a particular hospital (patients arriving at all nonreferral hospitals had similar wait times), nor as a result of delays in being admitted to a referral hospital (transfer patients were admitted quickly and were fast-tracked to surgery). The region-wide impact was a 1- to 2-hour increase in median wait times every half-year after the consolidation.
Time to surgery (log) for all patients.
Time to surgery (log) for Winnipeg Regional Health Authority (WHRA) patients.
Multiple linear regression confirmed these results (see the Appendix, Table S1). Both for the sample as a whole and more strongly for WRHA patients, there was a significant interaction between the intervention and the episode date. This indicated that time to surgery began to get longer after the intervention was implemented. Significant intervention effects were apparent at 12 and 18 months. There was also a significant interaction between the intervention and type of hospital: after the intervention, waits became longer at feeder hospitals and slightly and temporarily shorter at referral hospitals. When the analysis was restricted to patients who presented at referral hospitals (99% of whom remained there), no intervention effect appeared. To ensure that missing data for “time to surgery” had not biased our results, we performed a Poisson regression using days to surgery as the dependent variable. The effects for intervention and month as well as the interaction between them remained in the same direction and reached significance in the WRHA sub-sample (p = 0.041). The significant result is striking, considering the imprecision of the dependent variable, days to surgery, whose value was 0 or 1 for 72% of episodes.
Time to inpatient admission
Wait times for inpatient admission were difficult to interpret because the study period coincided with a trend toward admitting selected patients directly to the inpatient surgery ward, bypassing the ED. There was no apparent wait time for such patients, but this would seem to be an underestimate, since we do not know when they really presented in the system. To avoid underestimating postintervention wait times, we ran the analyses twice: once for patients who went through the ED and again for the full sample.
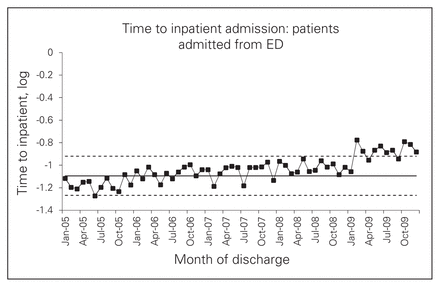
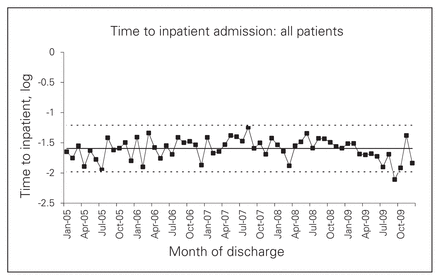
For patients admitted through the ED, SPC analysis showed an unmistakable increase in time to inpatient admission (Fig. 3). The most dramatic increase coincided with the jump in direct admissions, but some increase had already become apparent by early 2008, before the intervention period. However, the full-sample analysis suggested a possible decrease in wait times during the intervention period (Fig. 4).
Time to inpatient admission (log) for patients admitted from the emergency department (ED).
Time to inpatient admission (log) for all patients.
The regression models echoed the findings that after the intervention, wait times increased for admissions through the ED but decreased for admissions as a whole, although these effects varied in significance between WRHA patients and the full sample (see the Appendix, Table S2). As with time to surgery, the increased waits for admissions through the ED were more apparent for patients who presented to a nonreferral hospital (data not shown). Decreased waits for overall admissions were more apparent at referral hospitals, because only these hospitals adopted a policy of bypassing the ED.
These results imply that ACS patients who visited the ED postconsolidation spent longer there than they would have preconsolidation. However, an increasing number of ACS patients spent no time in the ED at all. The 2 trends may balance each other out, or it could be argued that 1 trend is more important than the other. It is difficult to draw overall conclusions without making assumptions about the journey of patients who do not visit the ED.
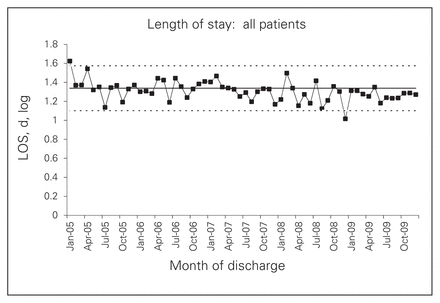
Length of stay
On control charts, LOS seemed slightly shorter after the intervention, but SPC analysis did not detect a significant change (Fig. 5). However, when the sample was split by operative status, this effect appeared only for nonsurgical patients, with no intervention effect for surgical patients (see the Appendix Figs. S5 and S6). In addition, the control chart for nonsurgical patients showed that any decrease in LOS began in late 2007, which was well before the intervention. Multiple regression revealed that LOS began to rise after the intervention, but again only for nonsurgical patients (see the Appendix, Table S3). These findings suggest that any variation in LOS did not imply a change in surgical outcomes.
Length of stay (log) for all patients.
Readmission, mortality and complications
Neither the SPC nor logistic regression detected an intervention effect on 30-day readmission rates, 30-day mortality or surgical complications (see the Appendix, Table S4). These outcomes occurred at virtually identical rates before and after the intervention (13.5% v. 13.5% for readmissions, 2.4% v. 2.5% for mortality and 6.0% v. 6.2% for complications). We also found that, compared with patients who presented at an ACS referral hospital, patients who transferred there did not have longer LOS or an elevated risk for complications, readmissions or death. A supplementary analysis revealed that the proportion of surgeries performed after hours did not change with the intervention.
Discussion
To our knowledge, this study is the first to examine wait times for ACS in a multi-hospital system, linking together episodes of care that were separated by transfers to measure the entire patient journey. The main limitations of this work, the lack of randomization and the inability to control for illness severity, are offset by the long interrupted time series design. This represents an advance over prior studies, which have featured simple pre–post or year-to-year comparisons. Through SPC, we were able to pinpoint the timing of observed effects, reducing the risk of bias from secular trends and unrelated developments.
The ACS intervention included both the region-wide consolidation of ACS and hospital-specific efforts to improve patient access through a designated ACS team. At the hospital level, strategies to improve access for ACS patients do appear to have helped. The new referral hospitals are handling a much higher volume of ACS patients than before, without increasing the amount of time between presenting at that hospital and receiving surgery. This achievement may be partly attributed to a reduced load of non-ACS patients, but may also reflect improved processes. There has also been a move toward streamlining inpatient admissions by allowing referred patients to bypass the ED. However, for the entire patient population served by the 6 hospitals, this study showed a longer average wait for access to emergent surgery following the ACS consolidation. The efficiency gains that may have occurred at the hospital level do not outweigh the extra time spent transferring patients who present at a nonreferral hospital.
The ACS intervention does not appear to have affected patient outcomes, with no changes in LOS, complications, readmissions or mortality. Thus, there is no evidence that the observed increase in wait times has harmed ACS patients. Since we relied on broad definitions of both the patient population and the outcomes of interest, it remains possible that we failed to detect certain positive and/or negative effects. In particular, since we were unable to control for severity of illness, we cannot determine whether the consolidation might have beneficially or adversely impacted the subgroup of complex and unstable patients (we would note that the policy of direct admission to a surgical ward does not apply to unstable patients, who are triaged to the ED; however, such patients may have been affected by other aspects of the consolidation). In addition, it is difficult to use LOS as a proxy for outcomes when the intervention involves shifting patients among hospitals that may differ in average LOS. Moreover, although our data included all of the most common ACS conditions, we may have missed some relevant diagnoses, and we found it too difficult to track the journey of patients whose ACS episodes were incidental to another hospital admission. In future, the creation of an ACS registry would permit the definitive identification of ACS patients. However, the best available data suggest that the consolidation did not affect broad outcomes across the ACS population.
This study did not track the potential impacts of ACS consolidation on non-ACS patients. Benchmarking data collected annually from 2007/08 through 2010/11 detected no significant changes in adverse outcomes (readmission, in-hospital mortality) for acute myocardial infarction or stroke patients at the referral hospitals, nor was there an increase in the number of hospital admissions for ambulatory care–sensitive conditions. These data provide no a priori evidence of patient harm, but firm conclusions cannot be drawn without more sensitive and frequent measures of emergency patient outcomes.
Conclusion
Our findings suggest that a regional ACS model can ensure adequate emergency surgical coverage without threatening patient outcomes. This makes it a viable solution to a health human resources problem that has become increasingly serious in Canada, the United States and elsewhere. Patient outcomes were not impacted; however, the growing number of patient transfers brought an overall increase in wait times. The WRHA has recognized a need to improve the current system so that all patients experience a smooth journey without delays; it is currently working to streamline the transfer process while also reducing surgical volume at referral hospitals by redirecting other types of surgery. The system is still evolving, and benefits may be realized as it develops. With time, ACS patients may gravitate and be directed toward the referral sites, with a lower proportion presenting at non-ACS facilities. What is clear, however, is that regionalization brings its own challenges, and evidence on the outcomes of within-hospital ACS consolidation cannot necessarily be generalized to the regional level. Returning to a model with unpredictable gaps in the emergency call schedule would not be an option for the WRHA, nor for other regions in similar situations; however, our findings do not provide grounds for wholesale adoption of the model regardless of context. It remains essential for jurisdictions that implement a regional ACS model to carefully monitor its impacts — both intended and unintended.
Acknowledgements
This research was undertaken as part of an evaluation of the Winnipeg Regional Health Authority (WRHA) Acute-Care Surgical Service. We thank Trevor Strome, Miroslava Svitlica, Jill Evison, Trudy Wilgosh, Ray Larkins, Cameron Robertson, Susan Gerlach, Wendy Rogocki, Evelyn Fondse, Anne Haknasson, Crystal Letain, Victor Cho and Elaine Pelletier for their help with data acquisition. We also appreciate the suggestions we received from the MNU/WRHA Working Group and from Dr. David Hochman.
Footnotes
Competing interests: None declared.
Contributors: S.A. Kreindler, C.J. Metge, R.W. Nason and M.E.K. Moffatt designed the study. L. Zhang acquired the data, which all authors interpreted and analyzed. S.A. Kreindler wrote the article, which all authors reviewed and approved for publication.
- Accepted November 14, 2012.