Abstract
Background: We sought to evaluate the adequacy of follow-up of thyroid cancer patients at a Canadian centre.
Methods: We mailed a survey to the family physicians of thyroid cancer patients and analyzed the findings relative to follow-up guidelines published by the American Thyroid Association (ATA). Statistical significance between early and late follow-up patterns was analyzed using the χ2 test.
Results: Our survey response rate was 56.2% (91 of 162). The time from operation ranged from 1.24–7.13 (mean 3.96) years, and 87.9% of patients had undergone a physical exam within the previous year. Only 37.4% and 14% of patients had a serum thyroglobulin measurement within 6 and between 6 and 12 months before the survey, respectively. Thyroid simulating hormone (TSH) levels were measured within the prior 6 months in 67% of patients and between 6 and 12 months in 13.2%. The TSH levels were suppressed (< 0.1 μIU/L) in 24.2% of patients, 0.1–2 μIU/L in 44% and greater than 2 μIU/L in 17.6%. Ultrasonography was the most common imaging test performed.
Conclusion: There is significant variation in the follow-up patterns of patients with thyroid cancer, and there is considerable deviation from current ATA guidelines.
The incidence of thyroid cancer has been steadily increasing over the past 3 decades.1,2 In the United States, the incidence in 1975 was 4.9 per 100 000 and increased to 12.0 per 100 000 in 2007, which represents a 2.4-fold increase.1 This has been largely attributed to increased detection of differentiated thyroid carcinoma (DTC), specifically papillary thyroid carcinoma (PTC).3 Differentiated thyroid carcinoma includes the histologic diagnoses of PTC, follicular thyroid carcinoma (FTC) and Hurthle cell carcinoma (HCC). Although thyroid cancer is relatively uncommon, it is the most common endocrine cancer and accounts for 95% of all endocrine malignancies.4 Prognosis for DTC is generally favourable, with its 5-year relative survival being 99.8% for localized disease and 97.1% when regional metastases to lymph nodes are present.5 However, thyroid cancer may recur and lead to morbidity and mortality as remotely as 30 years after primary disease treatment.6 Thus, patients with diagnosed thyroid cancer require diligent long-term follow-up and surveillance. In addition to disease surveillance, follow-up is important for monitoring thyroxine suppression therapy after surgical resection.7
There is controversy in the literature regarding follow-up and surveillance practices for thyroid cancer patients, with different guidelines published by different medical societies. Guidelines were initially published by the American Thyroid Association (ATA) in 1996 and revised in 2006 and in 2009 to assist physicians in developing an evidence-based treatment and follow-up algorithm for DTC.7 Other guidelines with different recommendations have been published by the National Comprehensive Cancer Network (NCCN) and the European Thyroid Association.8,9 With the rising incidence of DTC, it is essential to ensure that evidence-based practices for treatment and follow-up are followed and uniformly applied to ensure that patients with thyroid cancer are receiving appropriate medical care. Adequate follow-up practices facilitate early detection of disease recurrence and also ensure appropriate monitoring of TSH levels. Furthermore, adequate follow-up helps to avoid overly aggressive TSH suppression and its associated morbidities, including an increased risk of osteoporosis in postmenopausal women, or undersupplementation and the associated morbidities related to hypothyroidism.10 Previous studies have identified substantial variability in practice patterns regarding treatment and follow-up of patients with thyroid cancer.11–13 A study by Famakinwa and colleagues11 benchmarked national practice patterns in the United States against ATA guidelines using results obtained from cross-sectional analysis of surveillance, epidemiology and end results (SEER) for thyroidectomy, lymphadenectomy and radioactive iodine therapy of DTC.11 They reported significant variation in adherence to treatment guidelines by health care practitioners. These observations suggest that there is variation in the quality of care for DTC patients, especially elderly patients and minorities.11
At our institution, postsurgical follow-up of patients with thyroid cancer is not standardized, but it is always multidisciplinary. Surgeons provide initial short-term postsurgical follow-up, most patients are followed by an endocrinologist for a variable length of time postoperatively, and some patients may be followed by a radiation oncologist depending on the use of adjuvant radioactive iodine therapy and, less commonly, external beam radiation therapy. As is the case for most cancer types, at our institution the long-term follow-up and surveillance of patients with thyroid cancer is the responsibility of primary care physicians. Given the relative rarity of thyroid cancer and the complexities of its follow-up it is our hypothesis that there are limitations in the adequacy of postoperative follow-up and surveillance of this patient population. We have been unable to identify any prior studies that have examined “real world” follow-up practices for patients with DTC in Canada. The objective of this study was to determine whether individuals treated for DTC at our centre received adequate postoperative follow-up care in accordance with current guidelines.
Methods
We performed a retrospective, cross-sectional study of all individuals with diagnosed DTC larger than 1 cm who underwent an operation at a single tertiary care centre (St. Paul’s Hospital, Vancouver, BC) between 2003 and 2007. The histologic subtypes of PTC, FTC and HCC were included. We identified patients with thyroid cancer diagnoses through the hospital pathology information system, and the treating primary care physicians were identified through retrospective chart review. Our institutional research ethics board approved the study protocol.
In addition to providing first-line medical care, at our institution, primary care physicians facilitate specialist referrals, and collaborate with specialists and other health care professionals to provide comprehensive longitudinal health care and post-treatment follow-up for individuals in their care. Primary care physicians provide continuity of care and receive all consultation letters, clinical correspondences, test results and operative and treatment reports regarding their patients. Thus, at our centre, primary care physicians are responsible for providing long-term follow-up and surveillance and serve as a “home base” for patients receiving multidisciplinary cancer care. Therefore, for the thyroid cancer patient population treated at our centre, it is the primary care physicians who are ultimately responsible for their long-term follow up and have the most complete information on their long-term postoperative management. It was for these reasons that the primary care physicians were the source of follow-up information for the present study.
The primary care physicians of individuals who underwent surgery at our centre were contacted by mail with a survey package that consisted of a cover letter describing the study and a questionnaire. The survey packages were resent to primary care physicians who didn’t respond to the initial survey 6 months later to encourage a high level of study participation. The questionnaire requested information about their patients’ disease status (i.e., no recurrence, death from other cause, lost to follow-up); the timing of the most recent physical exam; results of laboratory investigations, including serum thyroglobulin (Tg) level, antithyroglobulin antibody (anti-Tg Ab) level, thyroid stimulating hormone (TSH), T4; and other relevant radiologic examinations, including ultrasonography, computed tomography (CT), diagnostic whole body radioiodine scan (DxWBS), magnetic resonance imaging (MRI) and positron emission tomography (PET). A description of the findings from the most recent physical exam; specific levels of Tg, anti-Tg Ab, TSH and T4; and the presence of normal or abnormal findings on the most recent imaging studies were also requested. Surveyed physicians were asked to specify whether Tg measurements were drawn while their patient was undergoing TSH suppression with thyroid hormone or while undergoing TSH stimulation through withholding levothyroxine or using recombinant human thyrotropin (rhTSH). We compared the survey results with recommendations from the ATA according to the revised management guidelines.7 Findings were also reviewed in the context of the local thyroid cancer follow-up recommendations published online by the British Columbia Cancer Agency (BCCA).14 The BCCA is responsible for establishing a coordinated, province-wide program of cancer control and management for residents of British Columbia and publishes online cancer management guidelines based on its accumulated experiences with best practice evidence derived from major cancer centres worldwide.14
Statistical analysis
All results were expressed as proportions of total patients. We used the χ2 test to compare follow-up patterns between early and later follow-up duration (less than 2 yr v. ≥ 2 yr). The BCCA recommends scheduled follow-up visits every 3–4 months for the first 2 years after treatment, every 6 months for the next 2 years after treatment if there is no evidence of recurrence, and annual visits thereafter.14 Early follow-up was therefore defined as less than 2 years from thyroid cancer treatment, as this was the time period with the most frequently recommended follow-up visits. The late follow-up time period was defined as 2 years or longer from thyroid cancer treatment. We compared patients who were followed for less than 2 years with those who received longer-term follow-up for 2 years or more after treatment. The frequency of follow-up testing based on the surveyed time intervals for each follow-up test were treated as categorical data. We assessed patterns of follow-up testing frequency using the the χ2 test. We considered results to be significant at p < 0.05. Statistical testing was 2-sided. We carried out statistical analyses using SPSS software.
Results
Of the 162 surveys mailed to primary care physicians, 91 (56.2%) were returned with adequate information for evaluation. Each survey was returned by a different primary care physicians. (i.e., no physician had more than 1 patient in the study population). Twenty (12.3%) surveys were returned with no information for analysis because the patient was deceased, the physician was unwilling to complete the survey, the patient was no longer in the care of the physician, or the patient had moved or was lost to follow-up. Seven (4.3%) surveys were returned undelivered because the primary care physician had moved his/her practice or retired, or because the survey was sent to the incorrect physician. The primary care physicians who completed the surveys answered most of the questions. We considered a survey to be adequate if a response was provided for more than two-thirds (66%) of the questions. Forty-four (27.2%) surveys were not returned.
The histologic type of DTC in all 91 patients for whom the survey was returned and adequate for evaluation was PTC. In all, 74 (81.3%) of these patients were women and 17 (18.7%) were men. The mean age of patients was 45.8 (range 21–75) years, and the mean MACIS (distant metastasis, patient age, completeness of resection, local invasion and tumour size) score was 4.81 (range 3.25–8.84) based on 88 of 91 patients; sufficient information to calculate the MACIS score was unavailable for the remaining 3 patients. Ninety surveys reported follow-up duration; the mean follow-up duration was 3.96 (median 3.66, range 1.24–7.13) years.
The disease status of the patients was recorded at the time the surveys were filled out by the primary care physicians. It was noted that 83 of the 91 (91.2%) patients were cured with no known recurrence; 3 (3.3%) had active thyroid cancer or cancer recurrence, 2 (2.2%) died from causes other than thyroid cancer and 2 (2.2%) were lost to clinical follow-up. The remaining survey (1.1%) did not include an answer to this question. The extent of thyroid surgery for the study population is summarized in Table 1. All individuals who underwent a thyroid lobectomy with a final pathological cancer diagnosis (n = 17) subsequently underwent completion thyroid lobectomy.
Thyroid operations that yielded cancer diagnosis
Physical examination
No specific recommendations have been made by the ATA regarding the appropriate frequency of physical examination after treatment of thyroid cancer. However, the BCCA recommends physical examination of the thyroid bed, lymph nodes of the neck and other symptomatic areas as a part of postoperative disease surveillance.14 Most of the study patients (67%) had undergone a physical examination within the 6 months before the survey, 20.9% between 6 and 12 months, 5.5% between 1 and 2 years, and 4.4% between 2 and 5 years. Two (2.2%) surveys had no response to this question. In their most recent physical exam, 74 (81.3%) patients had a normal physical exam with no evidence of recurrence and 3 (3.3%) patients had abnormal findings that were concerning for possible cancer recurrence (Table 2). These 3 patients specifically had a new neck mass identified. Two (2.2%) surveys had no responses.
Frequency of follow-up modalities expressed as percentage (%) of total patients*
Serum Tg and anti-Tg Ab measurements
According to the current ATA guidelines, serum Tg should be measured every 6–12 months after treatment of DTC (Recommendation 43 A).7 A serum Tg measurement was available for 68.1% of patients in our study. Only 37.4% of patients had a serum Tg measurement within the prior 6 months and 15.4% between 6 and 12 months after treatment, in accordance with the ATA guidelines. The remaining 29.7% of patients underwent serum Tg measurements less frequently than recommended (Table 2). No responses were available for this question for 17.6% of the patients.
According to the ATA, anti-Tg antibodies should be quantitatively assessed with every serum Tg measurement (Recommendation 43 A).7 Most patients (61.5%) in our study had at least 1 serum anti-Tg Ab measurement; 29.7% of patients had an anti-Tg Ab measurement within the prior 6 months, 12.1% between 6 and 12 months, 12.1% measured between 1 and 2 years, and 7.7% measured between 2 and 5 years. Roughly 30.8% of patients have never had an anti-Tg Ab measurement. No data were available for 7.7% of patients, and 53.8% had negative anti-Tg Ab levels (< 41 kIU/L).
The highest sensitivity for serum Tg measurement for identifying thyroid cancer recurrence is obtained following thyroid hormone withdrawal or stimulation using rhTSH.7 Nine of 91 (9.9%) patients in our study had serum Tg measurements with TSH stimulation either by withholding thyroid hormone or by administering rhTSH. Roughly 40.7% of patients had serum Tg measurements while their TSH was suppressed when receiving thyroid hormone. There were no responses to this survey question for 49.5% of patients.
The ATA recommends that for low-risk patients who have undergone remnant ablation and who have a negative cervical ultrasound and an undetectable TSH-suppressed Tg within the first year after treatment, serum Tg should be measured after thyroxine withdrawal or rhTSH stimulation approximately 12 months after the ablation to confirm absence of disease (Recommendation 45 A).7 These patients can subsequently be followed with yearly clinical examination and Tg measurements while on thyroid hormone replacement (B). Compliance with these guidelines could not be determined in our study because there was insufficient data to determine which patient risk stratification system was used by the treating physicians. In addition, remnant ablation therapy information was also not available for the study cohort.
TSH and T4 levels
One of the goals of long-term follow-up of thyroid cancer patients is to monitor thyroxine suppression or replacement therapy to avoid both under-replacement and overly aggressive treatment.7 Most study patients (86.8%) underwent a TSH level measurement; 67.0% of patients had a TSH measurement within the prior 6 months, 13.2% between 6 and 12 months, 4.4% between 1 and 2 years, and 2.2% between 2 and 5 years. Roughly 3.3% of patients never underwent a TSH measurement. No data were available for 9.9% of patients. Roughly 68.1% of patients underwent a T4 level measurement: 49.5% within the prior 6 months, 14.3% between 6 and 12 months, 3.3% between 1 and 2 years, and 1.1% between 2 and 5 years. No T4 levels were measured in 15.4% of patients, and T4 measurement data were not available for 16.5% of patients (Table 2).
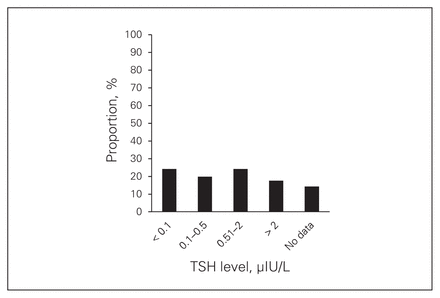
The ATA guidelines recommend that serum TSH may be kept within the low normal range (0.3–2 μUI/L) for individuals with no evidence of disease, especially those at low risk for recurrence (Recommendation 49 B).7 For higher-risk patients who clinically and biochemically have no evidence of disease, consideration should be given to maintaining TSH levels at 0.1–0.5 μUI/L for 5–10 years after treatment (Recommendation 49 C).7 For patients with persistent disease, the serum TSH should be maintained below 0.1 μUI/L indefinitely (Recommendation 49 B).7 In our study population, 68.1% of patients had serum TSH levels lower than or equal to 2 μUI/L, as recommended for low-risk patients who are free of disease, and 17.6% of patients had a serum TSH level above 2 μUI/L, which is higher than the minimum level of suppression recommended for disease-free or low-risk patients (Fig. 1). No data were available for 9.9% of patients (Fig. 1).
Thyroid stimulating hormone (TSH) and T4 levels in patients with thyroid cancer.
Neck ultrasonography
According to the ATA, cervical ultrasonography to evaluate the thyroid bed and central and lateral cervical neck compartments should be carried out 6–12 months postoperatively (Recommendation 48 B).7 Subsequent imaging with ultrasonography should be performed periodically depending on the patient’s risk of recurrent disease and Tg status (Recommendation 48 B).7 In our study it was difficult to determine whether ultrasonography was carried out in accordance with this guideline since the data collected were cross-sectional. However, more than half (57.1%) of the patients underwent cervical ultrasonography at least once: 25.3% of patients within the prior 6 months, 9.9% between 6 and 12 months, 11% between 1 and 2 years, and 11% between 2 and 5 years. Roughly 39.6% of patients did not undergo cervical ultrasonography, and data were unavailable for 3.3% of patients. Of the 52 patients who underwent ultrasonography, 84.6% had normal results and 13.5% had abnormal results; 3.3% of surveys did not provide information on ultrasonography results.
Diagnostic whole-body radioiodine scans
The ATA guidelines state that 6–12 months after remnant ablation, DxWBS may be useful for patients at high or intermediate risk for recurrent disease either after thyroid hormone withdrawal or rhTSH treatment (Recommendation 47 C).7 However, low-risk individuals who have had a first post-radioactive iodine remnant ablation whole body scan (WBS) with an undetectable Tg level and a negative anti-Tg antibody level as well as a negative neck ultrasound do not require routine follow-up DxWBS (Recommendation 46 F).7 In our study population 32 (35.2%) patients underwent a radioiodine scan and 56% did not; no response was provided in 8.8% of surveys. Of the 32 patients who underwent DxWBS, 27 (84.4%) had a normal result and 4 (12.5%) had an abnormal result (Table 2). No data were available for 8.8% of patients.
PET/PET-CT
According to the ATA guidelines, it is unlikely that low-risk patients require a PET scan as part of their initial follow-up.7 However, PET scans do play a role in localizing disease for Tg-positive (> 10 ng/mL) patients and patients with negative DxWBS (Recommendation 48 C).7 In addition, PET scans may be used as part of the initial staging of poorly differentiated thyroid cancers, as a prognostic tool for patients with metastatic disease, or to evaluate response following systemic or local therapy of metastatic or locally invasive disease (C). No patients in the present study were reported to have undergone PET.
CT and MRI
No specific recommendations have been given by the ATA regarding the role of CT or MRI in the postoperative follow-up of DTC patients. In the present study, 12.1% of patients underwent CT of the neck, and 82.4% did not; no data were available for 5.5%. Of the 11 patients who underwent CT, 9 (81.8%) had normal results and 2 (18.2%) had abnormal results.
In all 2.2% of patients underwent MRI and 82.4% did not; no data were available for 15.4% of patients. Both patients who underwent MRI had normal results.
Early versus late follow-up
There were no statistically significant differences when comparing early (< 2 yr from thyroid cancer treatment) to late (≥ 2 yr from thyroid cancer treatment) follow-up groups (Table 3).
Comparison of early (< 2 yr) versus later (≥ 2 yr) follow-up patterns using the χ2 test
Discussion
An important goal of long-term follow-up of thyroid cancer patients is to monitor for local disease recurrence and the development of regional or distant metastasis. No specific recommendations regarding the frequency of physical examination have been suggested by the ATA. The current BCCA guidelines recommend visits and evaluation by the most responsible physician every 3–4 months for the first 2 years after treatment, every 6 months for the following 2 years and annually thereafter.14 More frequent visits may be necessary if cancer recurrence is diagnosed or if there are complications. In the present study, 87.9% of patients underwent a physical examination in the year before the survey, in accordance with the minimum recommendation.
Without antibody interference, serum Tg has a high sensitivity and specificity for detecting thyroid cancer recurrence.7 A meta-analysis reported by Eustatia-Rutten and colleagues15 found that higher sensitivities for detecting disease recurrence were observed after total thyroidectomy and remnant ablation and that the highest sensitivities for recurrence were observed when measured after TSH stimulation using withdrawal of thyroid hormone therapy or rhTSH treatment than when measured with unstimulated postoperative Tg levels. About half (52.7%) of the patients in our study underwent an annual Tg measurement in accordance with the minimum recommended frequency by the ATA. A minority (9.9%) of patients underwent a serum Tg measurement with TSH stimulation. Anti-Tg antibodies should also be measured with every Tg level measurement, as they interfere with the immunometric Tg assay and spuriously lower serum Tg measurements.7 Only 61.5% of patients underwent at least 1 serum anti-Tg Ab measurement. The BCCA also recommends measurement of serum Tg as a useful marker for recurrence when levels rise in specimens drawn under the same conditions.14 A rising titre of anti-Tg antibodies may also suggest an increased risk of cancer recurrence.14
Several types of imaging tests are available for postoperative DTC disease surveillance. Ultrasonography has been shown to be superior to clinical examination for detecting local or regional recurrence and may be used to guide fine needle aspiration biopsy of lesions as small as 5 mm.16 The ATA recommends that cervical ultrasonography be carried out every 6 to 12 months after thyroid surgery, with subsequent ultrasonography carried out based on patient risk status and Tg level.7 It is difficult to determine whether ultrasonography was carried out in accordance with this guideline since the data collected were cross-sectional. However, 57.1% of patients in the present study underwent postoperative cervical ultrasonography at least once.
Whole body radioiodine scans have several limitations that impact their utility for long-term follow-up of DTC patients. Radioiodine ablation decreases the sensitivity of a WBS, and WBS provides little anatomic detail and has virtually no diagnostic yield in the setting of a negative Tg test result.16–18 The combination of ultrasonography and Tg measurement is more sensitive and specific for detection of local DTC recurrence than the combination of WBS and Tg measurement.19 The ATA does not recommend WBS for follow-up of low-risk thyroid cancer patients who have no other laboratory evidence of recurrence after remnant ablation.7 The BCCA guidelines specify that WBS should be continued until there is no evidence of uptake in the neck or elsewhere and then should be repeated only if the Tg level rises or if disease recurrence is detected clinically. In our study population 35.2% of patients underwent a radioiodine scan. However, it is unknown whether these were part of the initial post-ablation WBS or later DxWBS.
Positron emission tomography scanning has been used primarily to localize disease in Tg-positive, radioactive iodine scan–negative patients and patients with negative DxWBS.20 According to the ATA guidelines, it is unlikely that low-risk patients would require PET as part of their initial cancer surveillance.7 No patients in our study population underwent PET.
The availability, cost and accuracy of ultrasonography for detecting local and regional cancer recurrence, and the utility of PET/PET-CT for detecting WBS-negative local recurrence and metastatic disease, have led to limited use of CT and MRI for thyroid cancer surveillance.16 Furthermore, it is preferable to avoid the iodinated contrast agents used for CT in patients with DTC because they interfere with radioiodine scanning or therapy.16 However, CT may still have a role in detecting distant metastases, and MRI may be useful in evaluating invasion of adjacent structures.16 No specific recommendations have been given by the ATA regarding the role of CT or MRI in the postoperative follow-up of patients with a history of DTC. Only a minority of patients in our study underwent CT (12.1%) or MRI (2.2%).
A second goal of long-term thyroid cancer surveillance is monitoring of thyroxine suppression or replacement therapy to avoid under-replacement or overly aggressive treatment.7 Periodic TSH and T4 measurements are also recommended by the BCCA.7 Accordingly, 86.8% of patients in our study underwent a TSH level measurement, and 68.1% underwent a T4 level measurement. The TSH receptors are expressed on the cell membranes of DTC cells,21 and TSH stimulation leads to increased cell growth, invasion, angiogenesis, expression of thyroid specific proteins and inhibition of apoptosis of thyroid cancer cells.21–25 A meta-analysis reported by McGriff and colleagues26 suggested that suppression of TSH resulted in a significant reduction in major adverse clinical events, including disease progression, recurrence and death.26 In our study, 68.1% of patients had serum TSH levels lower than or equal to 2 μIU/L, as is recommended by current ATA guidelines for maintenance of patients who are free of disease, especially for those individuals who are at low risk of recurrence. Suppression to lower levels is recommended for individuals who are at a higher risk of disease recurrence or who have evidence of disease, though sufficient data are not available to determine how patients in the present study were risk stratified.
We compared early (< 2 yr after treatment) and late (≥ 2 yr after treatment) follow-up to determine whether follow-up practices deteriorated over time. There were no significant differences between these 2 groups in follow-up patterns identified with respect to frequency of physical examination, laboratory investigation or imaging.
Limitations
One limitation of the present study was that only 91 of 162 (56.2%) surveys were returned with sufficient information for analysis. This may reflect a nonresponse bias in which physicians with a particular interest in thyroid cancer follow-up or, conversely, physicians who may be less familiar with thyroid cancer follow-up or who have encountered more problems with follow-up procedures may have been more likely to respond to the survey. This resulted in a relatively small study population, which may not completely reflect the state of follow-up practices of patients who were surgically treated at our institution. In addition, not all surveys had complete answers for all the questions, and this may bias our results toward patients who received more complete follow-up. In addition, the data collected on follow-up were cross-sectional and did not allow evaluation of trends in follow-up over time for individual patients. Finally, information bias may also have limited our study because primary care physicians were the source of patient data, and we assumed that the primary care physicians had complete records on all procedures, tests and follow-up investigations for their patients. However, this assumption may have been incorrect; some information may not have been copied to the primary care physician, and follow-up investigations may have been carried out but the records may not have been available to the primary care physician. Such occurrences would bias our observations toward more incomplete follow-up trends.
A previous study by Van den Bruel and colleagues12 assessed thyroid cancer treatment and follow-up practices in Belgium through a survey that addressed the management of an index case with clinical variations and compared the responses with recommendations from the European Thyroid Association (ETA) Consensus Guidelines and ATA Guidelines. This study found variations in TSH suppression therapy practices for a good prognosis index case, and these differences reflected the differences in therapeutic goals proposed by the ATA and the ETA. The ETA recommends that TSH be maintained at ≤ 0.1 μIU/L, whereas the ATA recommends that the suppression level should be kept between 0.1 and 0.5 μIU/L.12 However, there was a clear indication for TSH suppression therapy in a poor prognosis case that was also presented, and the surveyed clinicians responded appropriately.12 In our study, there was insufficient data to risk-stratify the patients and thus determine their exact recommended level of TSH suppression. However, 68.1% of patients had serum TSH levels of 2 μIU/L of less, as is recommended for patients who are free of disease, especially those who are at low risk for thyroid cancer recurrence.
There may be barriers for primary care physicians to access certain tests, such as high-resolution ultrasonography of the neck, that may impact their ability to provide complete thyroid cancer follow-up. In addition, thyroid cancer is relatively uncommon, and educational opportunities in this area may be limited for primary care physicians. Educational interventions for primary care physicians, which include providing updated guidelines directly or in relevant journals and conducting educational seminars at appropriate conferences, are possible strategies that may improve primary care follow-up of patients with thyroid cancer in British Columbia and elsewhere. Another method for providing recommendations for thyroid cancer follow-up investigation may be through a standardized letter sent by the treatment team (ie. surgeon, endocrinologist, radiation oncologist) to the family physician after treatment is completed.
Conclusion
Our study suggests that there are considerable variations in follow-up practices for DTC patients who are treated at our centre. Currently, some individuals receive less intensive surveillance than recommended for the lowest risk population. Thus, these individuals are not undergoing adequate thyroid cancer surveillance in accordance with current guidelines. It is also interesting to note that 91% of patients with returned surveys were thought to be cured by their primary care physicians despite inadequate follow-up investigation. These results emphasize the need for more standardized and coordinated follow-up and surveillance practices and for better dissemination of information and education on follow-up guidelines for primary care physicians who provide care for thyroid cancer patients. Such strategies are currently underway at our centre. Given the current availability of evidence-based guidelines, prospective study of interventions that may improve follow-up of patients with thyroid cancer are warranted and may eventually lead to improved outcomes for individuals in whom this common endocrine malignancy is diagnosed.
Footnotes
Competing interests: None declared.
Contributors: S.M. Wiseman designed the study. E. Lam, S. Strugnell and S.M. Wiseman acquired the data, which E. Lam, C. Bajdik, D. Holmes and S.M. Wiseman analyzed. S.M. Wiseman wrote the article, which all authors reviewed and approved for publication.
- Accepted December 19, 2012.