Abstract
Background Two members from an academic tertiary hospital went to the National Cancer Institute in Tokyo, Japan, to learn how to perform an adequate D2 lymphadenectomy and to then introduce this technique in the surgical care of patients undergoing surgery for gastric cancer at a Western hospital. We aimed to compare the perioperative outcomes and long-term survival of Western patients who underwent gastric resection, performed by these 2 surgeons, before and after the surgeons’ short-course technical training in Japan.
Methods We conducted a retrospective comparative study of all patients (n = 27 before training and n = 79 after training) who underwent gastric resection for cancer by the same 2 surgeons between September 2007 and December 2017 at the Centre Hospitalier Universitaire de Québec — Université Laval (Québec, Canada). We collected data on patient demographic, clinical, surgical, pathological and treatment characteristics, as well as long-term survival and complications.
Results In the post-training group, the number of sampled lymph nodes was higher (median 33 v. 14, p < 0.0001), but this increase did not result in a higher number of histologically positive lymph nodes (p = 0.35). The rate of complications was lower in the post-training group (15.2% v. 48.2%, p = 0.002). The hospital stay was shorter in the post-training group (11 [standard deviation (SD) 7] v. 23 [SD 45] d, p = 0.03). The median survival was higher in the post-training group (47 v. 29 mo, p = 0.03).
Conclusion These results suggest that a short-course technical training in D2 lymphadenectomy, completed in Japan, improved lymph node sampling, decreased postoperative complications and improved survival of patients undergoing surgery for gastric cancer in a Western setting.
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death worldwide.1 In Canada, it was estimated that 4100 Canadians were diagnosed with gastric cancer in 2019, and 1950 died from it.2 Complete surgical excision is the only curative treatment for operable gastric cancer.3
Five-year survival for gastric cancer in Japan ranges from 92% for patients in stage IA to 35% for patients in stage IIIB. In comparison, 5-year survival in the United States ranges from 71% for patients in stage IA to 14% for patients in stage IIIB.4,5 In Eastern Asia, total or subtotal gastrectomy with D2 lymphadenectomy is the standard of care for the treatment of gastric cancer, with low rates of perioperative mortality and morbidity.6,7 There has been a debate over the need for D2 lymphadenectomy in gastric cancer patients in Western countries, both in terms of oncological impact and perioperative morbidity and mortality rates. Although both the Dutch8 and the Medical Research Council9 randomized controlled trials (RCTs), 2 major European trials published in the 1990s, initially showed higher mortality rates and higher frequencies of postoperative complications in a Western setting, another randomized trial from the Italian Gastric Cancer Study Group comparing D1 and D2 lymphadenectomy showed no difference in complications and death between the 2 groups.10 The 15-year follow-up results of the Dutch trial, published in 2010, showed lower locoregional recurrence and rates of death related to gastric cancer among patients who had undergone a D2 lymphadenectomy compared with those who had a D1 lymphadenectomy.11 In a 2006 RCT published by Wu and colleagues,12 comparing D1 and D3 lymphadenectomy, long-term results showed that even if D3 dissection was associated with higher morbidity, it did not lead to significant postoperative mortality rates and had a significant long-term survival benefit over D1 dissection. Finally, the recent Cochrane review by Mocellin and colleagues also showed that D2 lymphadenectomy could reduce the number of deaths due to disease progression as compared with D1 lymphadenectomy, though it showed no benefit when compared with D3 lymphadenectomy.13 The surgeon’s experience,14–16 the quality of the dissection17,18 and the necessity for appropriate training19 were all highlighted in recent literature as important features when performing D2 lymphadenectomy in a Western setting.
Considering recent publications on this subject, 2 members of our group went to Japan for a short-course, specialized, technical training to learn how to perform an adequate D2 lymphadenectomy. These surgeons then introduced this technique in the surgical care of patients undergoing surgery for gastric cancer at our institution. Therefore, the objective of the present study was to compare the perioperative outcomes and long-term survival of patients undergoing gastric cancer resection, performed by 2 surgeons, before and after the surgeons’ short-course training in Japan.
Methods
Study design and patients
We conducted a retrospective study of all consecutive patients, operated on by the 2 surgeons trained in Japan, who underwent gastric resection for cancer between September 2007 and December 2017 at the Centre Hospitalier Universitaire de Québec — Université Laval, an academic hospital in Québec, Canada. The study was approved by the ethics committee of the Centre Hospitalier Universitaire de Québec — Université Laval. The need for individual consent was waived by the committee.
We included patients with a pathological diagnosis of gastric adenocarcinoma, undergoing gastrectomy for cancer by 1 of the 2 surgeons trained in Japan, with a complete data set. The exclusion criteria were patients undergoing palliative resection and patients with a second primary cancer. The primary outcome was overall survival. The secondary outcomes were lymph node count and perioperative complications.
Training in Japan
Two members of our group of surgeons went to Japan in November 2013 for a 1-month short course of specialized technical training at the Cancer Institute Hospital of the Japanese Foundation of Cancer Research (Tokyo, Japan) and the Hyogo Cancer Center (Osaka, Japan). The objectives of this training were to learn the technique of Japanese D2 lymphadenectomy and to become familiar with the perioperative care of gastric cancer patients in Japan. This was an observership. The 2 surgeons observed 3 cases each day for 3 weeks, for a total of 45 cases.
Surgery
The extent of the gastrectomy was determined by the location of the tumour, with a targeted proximal margin of 5 cm. Before training, the type of lymphadenectomy used in our hospital was variable, ranging from D1 to D1+. All the patients in this study who underwent surgery after the training had a formal D2 lymphadenectomy according to the recommendations of the Japanese Gastric Cancer Association.7 The lymph nodes were found in the specimens by the pathologists.
Postoperative care
Many changes in the postoperative care of patients were made after the training in Japan. Patients were placed on an enhanced recovery program, with omission of a routine nasogastric tube and introduction of a liquid diet on postoperative day 1. Feeding tube jejunostomy was placed only for selected patients with severe preoperative malnutrition, compared with the more routine use of this approach pre-training. The consistency of the diet was progressed according to patient tolerance, resolution of postoperative ileus and nutritionist recommendations. Patients were encouraged to ambulate by the evening following the surgery, and all catheters were removed as soon as possible.
Follow-up
The routine follow-up after gastrectomy for cancer is 5 years. If no recurrence occurred during those 5 years, the patients were discharged from the hospital clinic and were followed by their general practitioner, who returned the patients to the clinic in case of suspicion of recurrence. For patients living far from the hospital, follow-up could be entrusted to a local hospital. We censored follow-up as of November 15, 2019. Because the patients were operated over 2 different time periods, we censored the follow-up in the pre-training group to the longest available follow-up in the post-training group. Therefore, the exposure time was the same in the 2 groups.
Data collection
We collected data prospectively in a database, including patient demographics, comorbidities, body mass index, tumour characteristics, clinical tumour site, lymph node involvement, metastasis (cTNM) stage, neoadjuvant or adjuvant treatments, use of diagnostic laparoscopy and cytology of the peritoneal lavage. Operative data included the procedure (total or subtotal gastrectomy, open or laparoscopic procedure), reconstruction, the inclusion of a feeding tube jejunostomy, operative time and estimated blood loss. Postoperative data included time to resume diet, length of hospital stay and postoperative complications according to the Clavien–Dindo scale.20 Pathologic data included surgical margins, lymph node count and tumour stage, including pathological TNM (pTNM) or post-therapy TNM (yTNM). Deaths and morbidity were obtained from the follow-up hospital chart.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed continuous variables were presented as means (standard deviations [SDs]) and analyzed using the Student t test. Non-normally distributed variables were presented as medians (ranges) and analyzed using the Kruskal–Wallis test. Categorical data were presented as frequencies and analyzed using the Fisher exact test. Survival was determined using the Kaplan–Meier method and analyzed using the log-rank test. All analyses were performed using SAS 9.3 (SAS Institute). Two-sided p values < 0.05 were considered statistically significant.
Results
Patient characteristics
Table 1 presents the characteristics of the patients. The post-training group showed a higher proportion of men (p = 0.03), and the tumour location pattern was different (p = 0.04). There were no significant differences between the 2 groups regarding other characteristics.
Patient demographic and clinical characteristics
Operative characteristics
Table 2 presents the operative data of the patients. In the post-training group, the number of reported lymph nodes was higher (median 33 v. 14, p < 0.0001), but it did not result in a higher number of positive lymph nodes found at histological examination (median 2 v. 1, p = 0.35), meaning that there was no stage migration. There was no significant difference in the numbers of node-positive cases (51.9% v. 67.1%, p = 0.17). Pre-training, 37.0% of the patients met the requirement for nodal dissection3 (16 lymph nodes), compared with 91.1% of patients post-training (p < 0.0001), showing that the quality standards changed after training. In the post-training group, a higher proportion of splenectomies was performed (33% v. 0%, p = 0.0002), as well a higher proportion of Roux-en-Y reconstructions (94% v. 48%, p < 0.0001) and stapled jejuno-jejunal anastomoses (p < 0.0001). There was no difference in the rate of diagnostic laparoscopies between the 2 groups. There was no difference between the 2 groups in the rate of positive surgical margins, operating time and blood loss. The approach to perioperative chemotherapy remained the same, but fewer patients received adjuvant radiotherapy in the post-training group (4% v. 19%, p = 0.02). The rate of complications was lower in the post-training group (15% v. 48%, p = 0.002). The length of hospital stay was shorter in the post-training group (11 [SD 7] v. 23 [SD 45] days, p = 0.03).
Patient operative characteristics
Survival
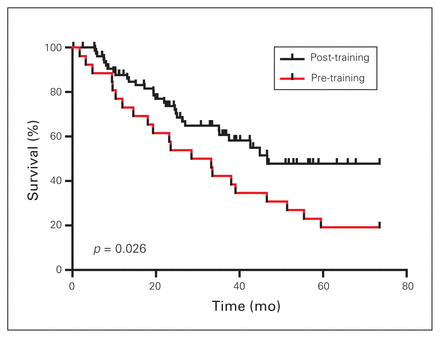
Table 3 presents the long-term survival of the patients. The median follow-up time was shorter in the post-training group (25 v. 39 months, p = 0.0001). The median survival was higher in the post-training group (47 v. 29 months, p = 0.03) (Figure 1). The 5-year survival was 19% and 48% in the pre- and post-training groups, respectively.
Overall survival of patients with gastric cancer before and after training in Japan (in months).
Patient survival
Discussion
The objective of the present study was to compare the perioperative outcomes and long-term survival of patients operated on by 2 surgeons before and after a short course of specialized technical training. The results suggest that an observership on D2 lymphadenectomy improved the extent of lymph node dissection and also improved the survival of gastric cancer patients in a Western setting, without compromising patient safety.
Surgery is recognized as the most important treatment option for gastric cancer, but outcomes have been disappointing in Western countries,21,22 although better survival has been reported in Eastern Asia.23,24 Differences in life habits, environment and genetics have been suggested, but Western surgeons historically performed a more limited lymphadenectomy than their Eastern Asian counterparts.25,26 In Eastern Asia, D2 lymphadenectomy is the standard of care for gastric cancer,6,7 and has been so in Japan for the last 50 years.27,28 Gastrectomy with D2 lymphadenectomy is a complex procedure associated with a steep learning curve29,30 and a high risk of postoperative morbidity and death, mainly because of splenectomy and distal pancreatectomy.10,11,31–33 Nevertheless, high-volume specialized centres can now achieve acceptable morbidity and risk.10,14–16,34,35
Expert surgical coaching has been described in recent literature, referring to when an expert surgeon imparts new skills or knowledge to another surgeon being coached.36 Its effectiveness has been shown, not only for medical students and residents, but for practising surgeons as well.37 Regarding expert coaching in D2 lymphadenectomy, Luna and colleagues30 recently reported lower morbidity and longer survival of patients who underwent D2 versus D1 lymphadenectomy after training in Japan, and highlighted the importance of appropriate training in a dedicated centre. There is also an ongoing educational project in Korea (KLASS-02-QC) that is aiming at standardizing D2 lymphadenectomy, highlighting the importance of the completeness of D2 lymphadenectomy.38 Another important aspect of surgeon education after residency is peer coaching, when 2 surgeons have a similar level of experience and knowledge and engage in a collaborative learning process. Peer coaching has been shown to be a good opportunity for continuous professional development for surgeons in independent practice, and peer coaching programs have already been implemented in the US.39 After attending the short-course training together in Japan, the 2 surgeons in this study assisted each other for most of the cases of gastrectomy and D2 lymphadenectomy, increasing each other’s exposure to the technique and contributing to an increase in the quality of the dissection. Finally, we believe that open surgery is still the gold standard approach in the Western setting, as D2 lymphadenectomy requires technical aspects that are very difficult to transpose in laparoscopy without high-volume exposure.
The postoperative care of the patients was modelled to the Japanese system7 and may explain, at least in part, the shorter length of stay and lower rates of postoperative complications. Early postoperative nutrition has been shown to reduce trauma-related high metabolism and help maintain the function of the intestinal barrier, hence decreasing the rate of gut-associated surgical infections.40 The benefits of this approach have already been demonstrated in gastric cancer patients.41 In the present study, the rate of complications was much lower after the training in Japan (from 48% to 15%), and we believe that standardized postoperative care played a major role in this improvement, particularly by decreasing perioperative deaths. Interestingly, more proximal tumours were treated with total gastrectomy and splenectomy in the post-training group, and more patients had pathologically positive nodes. Poorer survival would be expected in this situation, but the present study suggests the contrary. The changes in the surgical approach and postoperative care could, at least in part, explain this difference.
In Japan, the surgeons go to the pathology department after the surgery and examine the specimens themselves in order to determine the extent of lymph node sampling. In North America and Europe, as in our centre, the examination of the specimens for harvested lymph nodes is performed only by the pathologist. In the present study, the number of harvested lymph nodes was higher in the post-training group, and since the surgeons were not involved in the lymph node count, this suggests that the increase resulted from the new surgical approach applied after training.
Limitations
The present study has limitations. The sample size was small. The study spans a rather large period of time, and it is possible that changes in practice routine over time biased the results. Finally, the follow-up duration is different between the 2 groups, which could have affected the overall survival analysis. However, as gastric cancer is a lethal disease in the short term, the survival benefit (about 12 months) is significant. Prospective follow-up will be continued.
Conclusion
This study showed that short training in a specialized and experienced gastric cancer centre in Japan on D2 lymphadenectomy improved the extent of lymph node dissection and survival of gastric cancer patients in a Western setting. Emphasis should also be made on perioperative and postoperative care to decrease the rate of complications.
Acknowledgments
The authors acknowledge the invaluable contribution of Ann Wright, research and surgical ward nurse, for her help in data collection and maintaining the database.
Footnotes
The results have been presented as an oral presentation at the 2018 conference of the Société Française de Chirurgie (Paris, September 2018).
Competing interests: None declared.
Contributors: All authors conceived and designed the study, and acquired and analyzed the data. All authors wrote and critically revised the manuscript. All authors gave final approval of the article to be published.
- Accepted March 17, 2020.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/