Abstract
Background: The frequency with which patients with high Model for End-Stage Liver Disease (MELD) scores undergo liver transplantation has been increasing. Canadian literature regarding the outcomes of liver transplantation in recipients with high MELD scores is limited. The primary objective of this study was to assess patient and graft survival among recipients with high (> 35) and low (≤ 35) MELD scores. Secondary objectives were to potentially identify independent predictors of graft failure and patient mortality.
Methods: We conducted a retrospective chart review of patients undergoing liver transplantation at a single Canadian centre from 2012 to 2017.
Results: A total of 332 patients were included in the study: 280 patients had a MELD score of 35 or lower, and 52 had a MELD score above 35. Patients with high MELD scores had higher rates of pretransplant acute kidney injury and dialysis (p < 0.001), admission to the intensive care unit (ICU) or intubation (p < 0.001), intraoperative blood product transfusions (p < 0.001) and post-transplantation acute kidney injury and dialysis (p < 0.001), as well as longer ICU (p < 0.001) and hospital stays (p = 0.002). One- and 3-year patient survival in recipients with MELD scores of 35 or lower was 93.1% and 84.9% versus 85.0% and 80.0% in recipients with MELD scores above 35 (p = 0.37). One- and 3-year graft survival in recipients with MELD scores of 35 or lower was 91.7% and 90.9% versus 77.2% and 72.8% in recipients with MELD scores above 35 (p < 0.001). Prior liver transplant was an independent predictor of patient mortality, and no independent predictors of graft failure were identified. When MELD was replaced with D-MELD (donor age × recipient MELD), it predicted graft failure but not patient survival.
Conclusion: No difference in patient mortality was found between MELD groups. Graft survival was significantly lower in recipients with MELD scores above 35. D-MELD may potentially be used as an adjunct in determining risk of graft failure in recipients with high MELD scores.
In 2002, the United States introduced the Model for End-Stage Liver Disease (MELD) score as an organ allocation tool to help prioritize liver transplant (LT) candidates on LT wait lists.1,2 In 2004, provinces in Canada began to adopt the MELD score for organ allocation.3 Before the adoption of MELD, they primarily used the CanWAIT ranking system, which ranks patients by location (at home, in hospital, in the intensive care unit [ICU] or intubated in the ICU).3 An advantage of the MELD score over alternative scoring systems is that the points derived from the MELD score are strictly objective (international normalized ratio [INR], bilirubin and creatinine) whereas the Child–Pugh–Turcotte (CPT) score incorporates subjective assessments of hepatic encephalopathy and the amount of ascites (i.e., it essentially provides a nonobjective opinion of what is classified as mild, moderate and severe).
The MELD score was initially introduced to estimate 3-month survival for patients with end-stage liver disease (ESLD) awaiting a transjugular intrahepatic portosystemic shunt (TIPS) procedure.4 Subsequently, it has been validated as an accurate 3-month predictor of wait-list survival for potential LT recipients.5 Patients with MELD scores of 40 or above have an estimated 3-month wait-list mortality risk greater than 70%,6 and in practice these patients ultimately do not survive if they do not receive a transplant. Despite the MELD score’s important prognostic utility in predicting wait-list mortality, it is generally believed that it cannot predict post-transplant survival.7,8 Over the last decade the frequency with which patients with high MELD scores have undergone LT has increased, and with limited organ supply it will probably continue to increase.9
In 2015, Panchal and colleagues10 published the first US national multicentre outcomes report on patients with MELD scores of 40 and above undergoing LT. The authors found that these patients had inferior long-term graft survival (p < 0.001) and patient survival (p < 0.001) compared with patients with lower MELD scores; however, their outcomes were deemed to be acceptable. There is sparse published Canadian evidence of the post-transplant outcomes of LT recipients with high versus low MELD scores.11
The Canadian health care system differs from that of the United States, which may affect the availability of resources. Generalizing the US experience to Canadian centres may not be entirely appropriate, and studies of outcomes at Canadian centres with respect to this important issue are needed. A recent Canadian multi-centre retrospective study reviewed the baseline characteristics of critically ill patients with cirrhosis admitted to the ICU who underwent LT, although rates of graft failure were not available.12
In 2016, the Canadian Liver Listing and Allocation Forum recommended that the minimum acceptable estimated 5-year recipient survival rate be 60% for all deceased donor liver transplants.13 In the era of prioritizing the “sickest first” approach and the increased frequency of recipients with high MELD scores, analysis of the outcomes of Canadian recipients with high MELD scores is imperative to potentially improve post-transplant morbidity and mortality.
Methods
Patients
A retrospective cohort study was performed on all patients older than 18 years of age who underwent LT via donation after neurologic determination of death (NDD) or circulatory determination of death (DCD) between January 2012 and October 2017 at a single Canadian provincial LT centre, Vancouver General Hospital (VGH). Patients with acute fulminant liver failure or ESLD and those with a prior LT with graft failure who were awaiting transplantation were included. Patients with MELD exception points for pre-specified indications were also included. Live liver donor transplantation and combined multivisceral organ and partial liver graft transplantation were excluded from review. Ethics approval was obtained from the Clinical Research Ethics Board of the University of British Columbia.
Variables
Variables analyzed included baseline recipient characteristics, renal function (creatinine and glomerular filtration rate [GFR]) 3 months before, on the day of and after transplantation, and the need for preoperative or postoperative dialysis. Acute kidney injury (AKI) was defined as per the Acute Kidney Injury Network (AKIN) classification system.14 Chronic kidney dysfunction (CKD) was categorized into stages as per the Kidney Disease Improving Global Outcomes (KDIGO) classification system.15 Post-operative dialysis was defined as continued replacement therapy (CRT) or intermittent hemodialysis (IHD) within the first 7 postoperative days starting from the date of transplantation to capture the incidence of ongoing AKI from the pretransplantation period and new AKI secondary to the effect of the transplantation process itself, rather than AKI secondary to an insult that was not a direct consequence of the operation or its complications.
Data were collected on the American Society of Anesthesiologists (ASA) score for each patient, Can-WAIT status (status 1, chronic liver disease at home; status 2, chronic liver disease in hospital; status 3, chronic liver disease or acute liver failure in ICU not requiring ventilation; status 4, chronic liver disease or acute liver failure in ICU on a ventilator), MELD and Child–Pugh scores at the time of transplantation, portal hypertension, preoperative portal vein thrombosis, operative details (surgical technique, operative duration, blood products used, estimated blood loss), post-transplant complications (biliary, portal vein thrombosis and hepatic artery thrombosis, infectious complications) and ICU and hospital length of stay. Prior laparotomy was defined as any laparotomy that a patient had before their LT. For patients who underwent retransplantation, the initial LT was counted as having a previous laparotomy. Donor variables collected included age, sex and type of donation (DCD or NDD).
MELD score groups
High MELD score was defined as a score greater than 35 and a low MELD score was defined as a score less than or equal to 35, as supported by the findings from the retrospective multicentre Canadian study of critically ill patients with cirrhosis admitted to the ICU by Karvellas and colleagues.12 The authors reported that patients who underwent LT had a median MELD score of 34 and that recipient age older than 60 years was an independent predictor of 90-day mortality. LT recipients with MELD scores above 35 have also been found to have increased postoperative morbidity.16
Survival
Patient survival was calculated from the date of initial transplantation to patient death (due to any cause). If death did not occur, the patient was censored at their last known alive date. Patients with graft failure who survived were excluded from this analysis. Graft survival was calculated from the date of initial transplantation to the time of retransplantation (secondary to graft failure) or patient death (due to graft failure). If the patient did not have graft failure or died because of reasons unrelated to graft failure, they were censored at their date of death or last known alive date. The above analyses may not take into account competing events during a patient’s observation period. For instance, patient overall survival excludes patients with graft loss, and graft survival censors patients who did not survive, possibly affecting the results.
To properly account for these events in the survival analyses, we performed 2 additional competing risk assessments.17,18 Graft-censored overall patient survival was calculated from the date of initial transplantation to patient death. If death did not occur, patients were censored at their last known alive date. If patients had graft failure during their observation period, we also censored them at their retransplantation date as a competing event to death (instead of excluding these patients). Death-censored graft survival was calculated from the date of initial transplantation to the retransplantation date or patient death (due to graft failure). If graft failure did not occur, patients were censored at their last known alive date. If patients died because of reasons unrelated to graft failure during the observation period, we also censored them at their death date as a competing event to graft failure (instead of censoring as a nonevent).
Statistical analysis
Patient demographic and clinical characteristics were described using simple descriptive statistics (e.g., medians and proportions). To statistically compare the characteristics and the peri- and postoperative outcomes between the high-MELD and low-MELD groups, we used Kruskall–Wallis tests for continuous variables and χ2 tests of independence or Fisher exact tests (when expected counts were less than 5) for categorical variables. Kaplan–Meier curves with log-rank tests were computed to estimate the unadjusted patient overall and graft-free survival times stratified by high- and low-MELD groups. For graft- and death-censored survival, cumulative incidence curves with the Gray test were used.19 Numeric summaries for 1- and 3-year cumulative survival and incidence estimates and their associated 95% confidence intervals (CIs) were also derived. As a post hoc exploratory analysis, Kaplan–Meier curves with log-rank tests were computed to estimate graft and patient survival according to CanWAIT listing status.
Univariable and multivariable Cox proportional hazard models were employed to identify independent predictors for patient overall survival and graft survival. Potential variables were selected on the basis of a priori subject-area knowledge and pertinent literature. We included donor age, recipient age, donor type (DCD or NDD), previous LT, pretransplantation portal vein thrombosis, CanWAIT status (as an indicator of intubation status), ASA status, pretransplantation dialysis, pre-transplantation GFR and hepatitis C status. The level of significance was set at p < 0.05 for all statistical analyses, and all reported p values reflect 2-tailed tests. All analyses were conducted using R 3.5.2 statistical programming (R Core Team).
Results
Patient characteristics
Of 332 patients who underwent LT, 52 (15.7%) had MELD scores above 35 and 280 (84.3%) had MELD scores of 35 or below. Recipients with high MELD scores were younger (55.5 v. 57 yr, p = 0.044), more likely to be on dialysis from AKI (55.8% v. 4.3%, p < 0.001), more likely to be in the ICU and intubated (status 3 or 4) and more likely to have had a previous laparotomy and LT (Table 1).
Characteristics of patients who underwent liver transplantation
Intraoperative and postoperative outcomes
The high-MELD group required more intraoperative transfusions of packed red blood cells (7.50 v. 4.00 units, p < 0.001), fresh frozen plasma (10.00 v. 6.00 units, p < 0.001) and platelets (8.50 v. 3.00 units, p < 0.001) (Table 2). Sixty-two percent of transplant recipients with high MELD scores had a grade 3 post-operative AKI compared with 13.9% of recipients with low MELD scores, and they were more often initiated on dialysis (postoperatively) or continued on dialysis from before transplantation. LT recipients with high MELD scores had a significantly higher incidence of surgical site infections (17.3% v. 6.8%, p = 0.025) and fungal infections (21.2% v. 7.9%, p = 0.007), longer ICU length of stay (7.00 d v. 3.00 d, p < 0.001) and longer hospital length of stay (25.5 d v. 17.0 d, p = 0.002) (Table 3).
Intraoperative variables stratified by MELD score
Postoperative outcomes stratified by MELD score
Survival
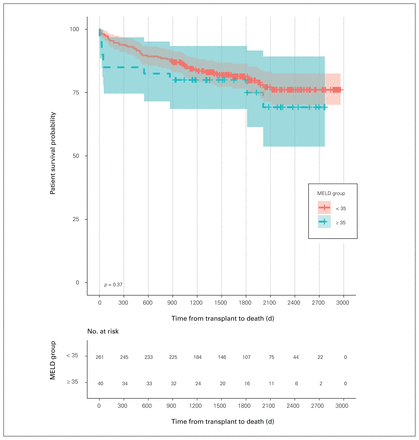
There was no statistical difference in patient survival between MELD groups (p = 0.37) (Figure 1). One-year survival among patients with MELD scores of 35 or lower was 93.1% versus 84.9% among those with scores above 35 (95% CI 74.6%–96.8%) and 3-year survival was 85.0% among patients with MELD scores of 35 or lower versus 80.0% among those with scores above 35 (95% CI 68.5%–93.4%). When failing grafts were included as competing events to death, there was no statistically significant difference in patient overall survival between the MELD groups for determining death (p = 0.77) (data not shown).
Patient survival. MELD = Model for End-Stage Liver Disease.
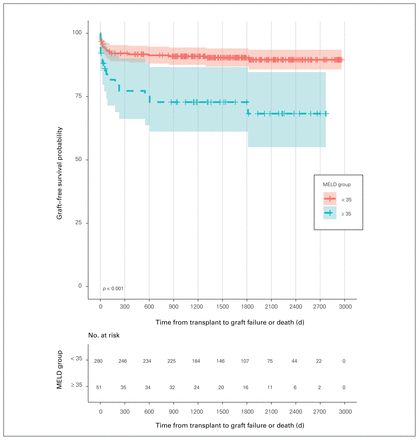
Overall, 27.5% of recipients with MELD scores above 35 experienced graft failure compared with only 10% of those with scores of 35 or lower. The high-MELD group had a significantly lower graft survival probability than the low-MELD group (p < 0.001) (Figure 2). One-year graft survival among patients with MELD scores of 35 or lower was 91.7% versus 77.2% among those with scores above 35 (95% CI 66.2%–90.1%), while 3-year graft-free survival was 90.9% versus 72.8% (95% CI 61.2%–86.7%). When death was included as a competing event to graft failure, MELD score above 35 continued to be statistically significant for predicting graft failure (p = 0.009) (data not shown). Patients with high MELD scores who experienced graft failure and did not die underwent retransplantation. Six patients with high MELD scores lost their first graft and underwent retransplantation for the following reasons: hepatic artery thrombosis (n = 1), ischemic cholangiopathy (n = 3), chronic rejection (n = 1) and portal vein thrombosis (n = 1). Five of the patients with high MELD scores who underwent retransplantation lost their second allograft because of uncontrollable bile leak and recurrent sepsis (n = 1), hepatic artery thrombosis (n = 2) and ischemia secondary to bleeding (n = 2).
Graft survival. MELD = Model for End-Stage Liver Disease.
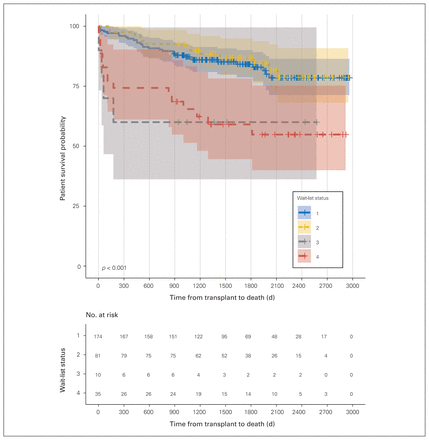
In an exploratory post hoc analysis, patient and graft survival was stratified by CanWAIT listing status; significant differences in patient survival (p < 0.001) and graft survival (p = 0.005) were identified (Figure 3). One- and 3-year patient survival were as follows: 94.8% and 86.6%, respectively, for status 1; 97.5% and 90%, respectively, for status 2; 60% for status 3; and 74.3% and 65.5%, respectively, for status 4. One- and 3-year graft survival were as follows: 90.4% and 89.9%, respectively, for status 1; 92.1% and 88.6%, respectively, for status 2; 58.6% for status 3; and 90.3% for status 4.
Patient survival stratified by CanWAIT listing status.
Graft survival stratified by CanWAIT listing status.
Univariable and multivariable analyses
Univariable analysis was performed to identify significant predictors for both patient death (Table 4) and graft failure (Table 5). Significant predictors for patient death were CanWAIT status 3 (hazard ratio [HR] 3.44, 95% CI 1.21–9.76, p = 0.021) and status 4 (HR 2.93, 95% CI 1.58–5.46, p = 0.001) relative to CanWAIT status 1, dialysis before liver transplant (HR 3.08, 95% CI 1.66–5.70, p < 0.001), history of prior liver transplant (HR 2.69, 95% CI 1.46–4.96, p = 0.02) and recipient age (HR 1.03, 95% CI 1.00–1.06, p = 0.022). Significant predictors for graft failure included CanWAIT status 3 (HR 5.04, 95% CI 1.88–13.52, p = 0.001) relative to CanWAIT status 1, dialysis for AKI and hepatorenal syndrome before LT (HR 3.68, 95% CI 1.87–7.25, p < 0.001) and MELD score above 35 (HR 3.22, 95% CI 1.69–6.15, p < 0.001).
Univariable analysis predicting patient death
Univariable analysis predicting graft failure
We performed multivariable analyses including all of the predictors selected a priori for patient death (Table 6) and graft failure (Table 7). For patient death, the only significant predictor was previous LT (HR 2.99, 95% CI 1.09–8.19, p = 0.033); CanWAIT status 3 (HR 3.42, 95% CI 0.98–11.9, p = 0.05) and CanWAIT status 4 (3.51, 95% CI 1.00–12.3, p = 0.05) approached significance. No variables predicted graft failure, although MELD score above 35 approached significance (HR 3.38, 95% CI 0.91–12.60, p = 0.07).
Multivariable analysis estimating patient death
Multivariable analysis estimating graft failure
There was no significant difference in the incidence of bile leak, biliary strictures or hepatic artery thrombosis between recipients with high and low MELD scores who received DCD grafts. There was no significant difference in recipient or graft survival when DCD and NDD donors were compared (data not shown).
Discussion
To our knowledge, this is the first Canadian study to report granular outcomes comparing transplant recipients with high versus low MELD scores. Recipients with high MELD scores had a higher incidence of pre-transplant AKI requiring dialysis, had a higher likelihood of requiring ICU admission and mechanical ventilation, and required higher usage of intraoperative blood products. The increased morbidity of recipients with high MELD scores resulted in an increased requirement for post-transplant dialysis, longer stays in the ICU, a higher incidence of fungal infections and surgical site infections, prolonged hospital admission and a higher rate of graft failure. It has been previously demonstrated that recipients with high MELD scores are more likely to require mechanical ventilation, increased intraoperative blood product utilization and vasopressor support.20 The increased morbidity and hospital resource allocation of recipients with high MELD scores has also been shown to lead to increased costs per patient.21
In the current study, the survival of patients with MELD scores above 35 was similar to that of patients with MELD scores of 35 or below, and these results correlate with what has been reported in the literature.22,23 Graft failure occurred more frequently in patients with higher MELD scores mainly becauase of arterial and biliary complications. It is difficult to know why these complications in particular had such a significant impact on patients with high MELD scores, but it is safe to assume that “sicker” patients physiologically do not tolerate such complications well. Alternatively, these complications are more likely to occur in the pathophysiologic milieu of a very ill patient. Regardless, our findings provide an opportunity to attempt to improve future graft survival by reducing postoperative arterial and biliary complications.24
It is also possible that some patients with higher MELD scores may have received less than optimal grafts that would have otherwise not been used or would have been allocated to candidates with lower MELD scores judged to be better able to tolerate a suboptimal graft. This situation may have occurred if the transplant surgeon and hepatologist believed that the patient with a high MELD score was likely to die before another graft became available. This risk versus benefit decision-making perhaps warrants further qualitative study. Furthermore, geographic distance remains a potential challenge within the Canadian transplant system for patients with high MELD scores. Unlike in the United States, there is no set MELD score that triggers eligibility for organs from outside the patient’s listed region. Owing to the geography and the relatively small population of some regions, such patients may wait an unacceptably long time if they are eligible only for organs from their own organ procurement organization. Therefore, transplant centres may be forced to use marginal grafts for patients with high MELD scores at times, resulting in poor outcomes. This is a topic that will need further discussion at the national level to ensure that patients with high MELD scores who have the highest wait-list mortality can undergo transplantation with an organ of appropriate quality in a timely fashion.
The lack of significance in graft failure rates between MELD groups who received DCD grafts and the lack of significance in graft failure rates between DCD and NDD donors is probably a reflection of a small sample size. Transplants from DCD donors have been associated with increased biliary complications, graft loss and recipient mortality when compared with those from NDD donors.25–27 Additionally, early allograft dysfunction from DCD donors has been shown to result in worse graft and patient survival.28
When survival was analyzed according to CanWAIT listing status, there was a significantly higher risk of 1- and 3-year mortality for status 3 patients (60.0%) and status 4 patients (74.0% and 65.0%). For graft survival, only status 3 patients had significantly worse outcomes. For both status 3 and status 4 groups, analysis was limited to small numbers: 13 status 3 patients and 38 status 4 patients. This probably reflects the lack of statistical significance of status 3 or status 4 patients predicting patient death or graft failure in the multivariable analysis. Multivariable analysis identified only a history of previous LT (HR 2.99) as a significant predictor of patient death. CanWAIT status 3 (HR 3.42), status 4 (HR 3.51) and preoperative dialysis (HR 3.35) nearly reached significance. No variables on multivariable analysis were significant predictors of graft failure; however, MELD score above 35 approached significance.
The minimum acceptable 5-year estimated patient survival for all indications according to the Canadian Liver Listing and Allocation Forum is 60%.13 Although we report only 1- and 3-year patient survival, our high-risk recipient outcomes overall meet the 60% survival threshold. Additionally, surviving the first year of transplantation has been previously shown to be a surrogate marker of good long-term survival.29,30
Panchal and colleagues10 and Nekrasov and colleagues9 reviewed the United Network for Organ Sharing (UNOS) database for LT recipients with MELD scores above 40; they each reported 1- and 3-year graft survival rates of 77% and 69% along with 1- and 3-year mortality rates of 80% and 72%. On multivariate analysis, Nekrasov and colleagues identified independent risk factors for graft failure: previous LT (HR 1.63), ventilator dependence (HR 1.52), hepatitis C infection (HR 1.42), pretransplant diabetes (HR 1.38), age older than 60 years (HR 1.32), waiting more than 4 weeks (HR 1.22) and prior abdominal surgery. Independent risk factors for recipient mortality included previous LT (HR 1.61), ventilator dependence (HR 1.49), diabetes (HR 1.47), hepatitis C infection (HR 1.46), age older than 60 years (HR 1.43), hospital admission time and prior abdominal surgery. Both studies had much larger study populations of 2610 and 5002 patients, respectively, and both groups defined high MELD score as 40 and above. The slightly higher 1- and 3-year graft and patient survival in our study is probably attributable to our definition of high MELD as a score above 35 rather than 40 and above.
Asrani and colleagues30 in 2017 developed a point-based risk score assessment tool based on the Scientific Registry of Transplant Recipients (SRTR) database. Ventilator support was awarded 5 points, recipient age older than 60 years and preoperative dialysis were awarded 3 points, and preoperative diabetes and preoperative creatinine greater than or equal to 1.5 mg/dL were awarded 2 points. Five-year patient survival and graft survival were greatly reduced in patients with more than 8 points compared with fewer than 4 points. Rana and colleagues8 and Dutkowski and colleagues31 have also identified previous LT and life support before LT among other variables as independent predictors of post-transplant survival. Each group developed their own risk stratification tools (survival outcomes following LT [SOFT] and balance of risk [BAR]) in the hope of optimizing donor allocation and patient outcomes. Both tools incorporate donor and recipient variables.
To help predict patient survival after LT, Halldorson and colleagues32 developed the D-MELD score (recipient MELD score multiplied by donor age). The authors reviewed 17 942 patients from the UNOS database between 2003 and 2006 with chronic liver disease who underwent LT. In patients with a pretransplant MELD score of 30 or above and a D-MELD score of 1600 or above, 4-year survival was 63.8% versus 71.3% in patients with a D-MELD score below 1600. De Boer and colleagues33 reviewed several predictive models for graft and patient survival in 62 294 patients from the Scientific Registry of Transplant Recipients database between 2005 and 2015. The D-MELD had a c-index (predictive capacity) of 0.58 and 0.56 for 1- and 3-year patient survival, respectively. The Donor Risk Index (DRI) score, which excludes recipient variables, has not been shown to be helpful in predicting mortality in recipients with high MELD scores.21
Owing to the simplicity of the D-MELD calculation and its inclusion of donor age, we aimed to see if it could predict patient survival in our cohort in a post-hoc analysis. We replaced MELD with D-MELD on multivariable analysis for predicting patient death and graft failure. D-MELD did not have any significance in predicting patient death, although it was statistically significant in predicting graft failure for recipients with MELD scores above 35 (HR 1.0, 95% CI 1–1, p = 0.014). Interestingly, De Boer and colleagues33 found that D-MELD had a poor c-index for graft failure. Our multivariable analysis of recipients with MELD scores above 35 did not predict graft failure (p = 0.069) and on univariable analysis donor age was not significantly different between MELD groups. Despite the lack of ability of either recipient MELD or donor age to independently predict graft failure, perhaps the D-MELD calculation can be used as an adjunct in determining which subset of patients with high MELD scores would be at high risk for graft failure.
In addition to survival analysis, we performed a competing risk analysis to account for patients who suffered graft loss in mortality estimates or for patients who did not survive in the graft failure analysis. We found that there was no significant difference between Kaplan–Meier and competing risk analysis for both patient survival and graft survival. In our study, 13 of 52 (25%) of the patients with high MELD scores had a prior liver transplant. Of the 24 patients with high MELD scores who had a prior laparotomy, 13 had a prior liver transplant. Thirteen of the 24 patients with high MELD scores with a prior laparotomy may have derived a high MELD score secondarily to complications associated with a failed liver transplant rather than a pathophysiologic decompensation of the underlying chronic liver disease or acute fulminant liver failure. The most common indication for laparotomy aside from previous transplant was emergency ventral or umbilical hernia repair in 4 patients. Other indications included exploratory laparotomy in 2 patients and subtotal colectomy for toxic megacolon, open cholecystectomy, liver resection, open liver ablation and cesarean section in 1 patient each.
Limitations
The limitations of the study include its retrospective design, a relatively small number of LT recipients with high MELD scores (> 35) and the inclusion of patients who underwent retransplantation, which may limit the generalizability of our results.
Conclusion
Higher MELD score was associated with lower graft survival but not patient survival after LT in this single-centre study. The increased morbidity associated with high MELD scores among recipients led to increased ICU and hospital length of stay. A history of previous LT independently predicted patient mortality, while in the group of patients with MELD scores above 35 the D-MELD score independently predicted graft failure. Even though patients with high MELD scores had increased morbidity than patients with lower MELD scores, patient survival was similar in the 2 groups. Collaboration with other Canadian centres may lead to improved identification of independent risk factors for graft failure and patient survival. Additionally, this may lead to improved selection of recipients with high MELD scores, optimization of donor allocation and informed consent for this high-risk patient population.
Footnotes
Competing interests: V. Marquez has received personal fees from Abbott Laboratories and Intercept Pharmaceuticals and support for attending meetings and/or travel from Intercept Pharmaceuticals. He has participated on a data safety monitoring board or advisory board for AbbVie, Paladin Labs, Eisai and Lupin Pharma. E. Yoshida has been an investigator for clinical trials sponsored by Gilead Sciences, AbbVie, Merck, Genfit, Pfizer, Intercept Pharmaceuticals, Calgene, Novo Nordisk, Allergan and Madrigal Pharmaceuticals. He has received an unrestricted research grant from Paladin Labs and speaker fees from Gilead Sciences Canada, AbbVie Canada and Merck Canada. S. Chartier-Plante has received consulting fees from Paladin Labs. No other competing interests were declared.
Contributors: M. Bleszynski, C. Scudamore, A. Buczkowski and P. Kim conceived the study. S. Punnen, E. Yoshida, S. Jayakumar, M. Segedi, A. Buczkowski, acquired the data, which S. Desai, T. Hussaini, V. Marquez, S. Chartier-Plante, S. Chung, A. Buczkowski, analyzed. M. Bleszynski, S. Punnen and P. Kim wrote the article, which S. Desai, T. Hussaini, V. Marquez, E. Yoshida, S. Jayakumar, S. Chartier-Plante, M. Segedi, C. Scudamore, S. Chung and A. Buczkowski revised critically for important intellectual content. All authors approved the final version to be published.
- Accepted August 10, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/