Abstract
Penetrating cardiac injuries require rapid diagnosis, efficient exposure and nuanced technical approaches, within a framework of highly coordinated and integrated multidisciplinary care. Acute care surgeons, with both strategic and technical expertise, are ideally positioned to address the potentially devastating consequences of these injuries. The aim of this narrative review is to offer a technical approach to the rapid evaluation, exposure, operative repair and postoperative care of penetrating cardiac injuries. A comprehensive review of the cardiac trauma literature, dating back to 1970, has provided a detailed toolbox of approaches to subxiphoid pericardial windows, resuscitative thoracotomy, median sternotomy, pericardiotomy, aortic clamping, cardiac hemorrhage control, cardiac repair, coronary artery injuries, pericardial closure, drain placement, chest wall closures, damage control thoracic procedures and immediate postoperative cardiac care, all based on fundamental physiological principles and anatomical considerations.
Cardiac injuries are often challenging from both physiological and anatomical points of view. Rapid preoperative decision-making, based on bedside imaging and clinical acumen, is essential. Technical excellence and nuanced postoperative critical care is also central to saving patients. This narrative review summarizes literature on operative strategy and tactics in penetrating cardiac trauma, to heighten and refine the preparedness of acute care surgeons and their teams for an injury that tests the functionality and integration of trauma systems.
First principles
Team dynamics and responsibility
Success in the management of penetrating cardiac injuries depends on the presence of predefined systems of trauma care capable of moving patients quickly, accessing necessary resources (e.g., blood bank) and equipment (e.g., resuscitative thoracotomy tray [Figure 1], operating room), and seamlessly integrating the expertise of multidisciplinary teams in a multimodal diagnostic and therapeutic response. Acute care surgeons are well positioned to lead teams in the care of patients with penetrating cardiac injuries, with their ability to make adaptive decisions under conditions of uncertainty and time dependence, understanding of damage control physiology and strategy, broad technical skills and familiarity with principles of crisis resource management. With some preparation, all acute care surgeons are capable of taking decisive steps toward achieving good surgical exposure, release of tamponade and hemorrhage control. During deployment of initial operative and resuscitative strategies, the acute care surgeon will often have to rapidly and concurrently assemble, sequence and integrate the contributions of a team of consultants (e.g., anesthesiologists, cardiac surgeons, critical care physicians, referral centres), depending on the nature and complexity of the injury.
The set. Trauma teams should be deliberate and parsimonious about what is included in the emergency room thoracotomy tray, to help streamline the operative intervention. Essential instruments (in the usual order of use) include a no. 10 blade scalpel (which is often carried by trauma surgeons or taped to the outside of the tray), curved Mayo scissors, a Finochietto retractor, a Lebsche knife and mallet, Allis clamps, Metzenbaum scissors, needle drivers, DeBakey forceps, monofilament sutures (e.g., 2-0 or 3-0 polypropylene sutures on MH tapered 3/8 circumference needles), a DeBakey aortic cross clamp and a Satinsky clamp.
Coordination between the operative and resuscitative teams must be seamless. Teamwork determines the pace and timing of transfusions and the adequacy of cardiac compressions, as well as the administration of resuscitative adjuncts such as calcium chloride to support cardiac contractility and vasomotor tone, adenosine to facilitate cardiac repair, epinephrine as a bridge for hemodynamic support, magnesium and defibrillation for arrhythmias and sodium bicarbonate to mitigate the metabolic effects of reperfusion.
Initial assessment
All penetrating thoracic injuries should trigger a high degree of suspicion for penetrating cardiac injuries. In modern trauma assessments, the classic findings of cardiac tamponade, such as Beck’s triad (muffled heart sounds, jugular venous distension and hypotension), electrical alternans and pulsus paradoxus (a fall in systolic pressure, > 10 mm Hg during inspiration), have been eclipsed by the use of focused assessment with sonography for trauma (FAST) ultrasonography to determine presence of pericardial fluid, often supplemented by correlates found by extended FAST such as inferior vena cava distension. If the initial assessment is suggestive of penetrating cardiac injuries, the patient’s hemodynamic status determines the location and invasiveness of subsequent diagnostic and therapeutic efforts, which can range from a subxiphoid pericardial window or urgent operative exploration in the operating room, to a resuscitative thoracotomy in the emergency department.
Anatomical considerations
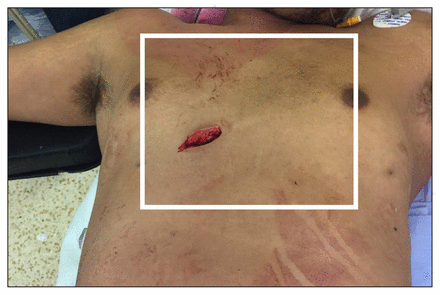
Detailed knowledge of precordial anatomy is critical to the successful management of patients with penetrating cardiac injuries. Mediastinal wounds should be suspected with any clinical evidence of trauma to the cardiac box (i.e., the space bordered by the midclavicular lines, clavicles and costal margins) (Figure 2). However, the observation that a higher mortality rate has been observed for cardiac trauma associated with wounds outside the box highlights the importance of maintaining a high index of suspicion for cardiac injury for all penetrating thoracic trauma.1 As a result, it is essential that clinicians consider both the specific mechanism and potential trajectory of a given injury pattern. Special attention should be given to gunshot wounds, as they often inflict substantial blast injury along their trajectories.2
The cardiac box. The surface anatomy of penetrating injuries can guide the operative approach. For example, a stab wound in the cardiac box (which is bound by the clavicles above, the mid-clavicular or nipple lines laterally and a horizontal line between the costal margins below), should suggest an anterior cardiac injury, until proven otherwise, and may influence the trauma team to access the heart in a patient in stable condition with cardiac trauma with a median sternotomy. Conversely, penetrating thoracic injuries outside of the cardiac box may suggest associated noncardiac thoracic injuries that may be better accessed from an anterolateral thoracotomy.
The palpable sternomanubrial joint should first be identified to locate the second rib and its corresponding intercostal spaces. The manubrium itself resides cephalad to this joint. The cartilaginous xiphisternum is connected distally, and therefore past the main sternal body that overlies a large portion of the anterior heart. The most commonly injured chamber of the heart ( > 50 % of penetrating cardiac injuries) is the right ventricle owing to its anterior location, which spans vertically between the third costal cartilages and the inferior cardiac border at the xiphisternal joint. The right border of the heart, which consists mostly of the right atrium between the vena cava, lies parasternally between the third and sixth costal cartilages. Conversely, the left border of the heart is formed mainly by the patient’s left ventricle, descending from the second parasternal intercostal space to the fifth intercostal space at the midclavicular line. Injuries to the left lateral border are thus more lethal owing to the high-pressure system it encloses.3 An oblique line between the medial portion of the third intercostal space and the cardiac apex roughly traces the anterior interventricular groove where the left anterior descending artery resides (often within a fat pad). Injuries to this region mandate further planning and assessment for a potentially lacerated coronary artery and subsequent cardiac ischemia.
The posterior surface of the heart begins at the T4–T5 intervertebral level where the arch of the aorta resides and the trachea bifurcates. The heart then extends caudally across the mediastinum to the T8–T9 intervertebral level where it rests on the diaphragm.
Technical considerations
Subxiphoid pericardial window
In patients with suspected penetrating cardiac injuries, the FAST examination, which screens for pericardial fluid, has been shown to have superb test performance, while also improving both the time to definitive care and overall survival.4 However, the sensitivity of bedside ultrasonography in detecting pericardial fluid can be limited by concomitant hemopneumothorax, synchronous lacerations to the pericardial tissue or pleura, subcutaneous emphysema or operator inexperience. Although it is a traditional test with vanishing indications, for patients in stable condition with suspected penetrating cardiac injuries and equivocal FAST findings, the subxiphoid pericardial window remains a powerful and definitive diagnostic adjunct. A recent randomized trial involving patients in stable condition with suspected penetrating cardiac injuries and ultrasonography-detected hemopericardium, compared an approach using a subxiphoid pericardial window, gentle pericardial irrigation and pericardial drain placement, with the more traditional and time-honoured approach of immediate exploration by way of median sternotomy, showed that the subxiphoid pericardial window approach is safe and effective for patients who remained in stable condition and in whom the pericardial irrigation returned clear.5 Thus, performance of a subxiphoid pericardial window still has a specific and important role in the management of penetrating cardiac injuries that do not mandate immediate sternotomy.
The subxiphoid pericardial window can be performed in the emergency department, intensive care unit or the operating room under general anesthesia. A 5–6 cm vertical, midline incision is centred over the xiphoid process. Deeper tissues are then separated by electrocautery or a spreading technique with scissors. Placing patients with larger body habitus in the Trendelenburg position will assist in facilitating exposure to the xiphisternum and pericardium.6 The linea alba is carefully incised without breaching the peritoneal cavity. The xiphoidectomy is completed by releasing the tissue around the xiphoid process with either electrocautery or Mayo scissors. Alternatively, the xiphoid can be hinged upwards (i.e., similar to opening the hood of a vehicle). The distal sternum is retracted anteriorly and careful dissection is engaged toward the pericardiodiaphragmatic junction. It may be necessary to divide some of the anterior diaphragm before the pericardium can be identified. When initial visualization is poor despite dissection of the surrounding tissue, which is often, digital palpation of the cardiac impulse can be used as a guide to locate the pericardium. Use of a sponge stick to push the precordial fat out of the way laterally in a corkscrew movement is also very helpful. The heart is further revealed by pushing down the inferior diaphragm. The pericardium is then grasped tautly with 2 long Allis clamps. A vertical 1–2 cm incision is made in between the 2 clamps with a no. 15 scalpel blade (or sharp scissors) to reveal, in the absence of penetrating cardiac injuries, a trace of clear pericardial fluid and the underlying epicardium. It is essential to ensure a completely bloodless field (ideally with white surgical sponges) before breaching the pericardium. A false-positive result, secondary to contaminated blood from outside the pericardial space, is suboptimal. A positive subxiphoid pericardial window is noted by the evacuation of a clot or blood staining within the pericardial fluid. The pericardial sac is then irrigated with warm saline to confirm any active bleeding. Communication with an anesthesiologist is pertinent as drainage can immediately reduce preload and trigger hemodynamic collapse in some patients. Progressive deterioration during the procedure may mandate an emergency sternotomy or thoracotomy. A positive subxiphoid pericardial window is classically followed by a median sternotomy and pericardiotomy. A pericardial window can also be created transabdominally during the course of an exploratory laparotomy, if a cardiac injury is suspected. The falciform ligament is traced superiorly to its diaphragmatic reflection near the confluence of the hepatic veins. A spot to the left of the falciform on the undersurface of the central tendon of the diaphragm is grasped and elevated between 2 Allis clamps. A vertical incision is made on the diaphragm between the clamps and feathered down toward the pericardium. Again, hemostasis should be maintained to avoid a false-positive result when the pericardium is opened.
Left anterolateral thoracotomy and clamshell thoracotomy
Patients with penetrating cardiac injuries presenting with no pulse or hemodynamic instability (i.e., despite fluid resuscitation or cardiopulmonary resuscitation, < 15 min) require resuscitative thoracotomy, often by way of a left anterolateral thoracotomy (LAT).7 The LAT approach offers rapid access to the heart and left thoracic structures. This also allows for timely decompression of cardiac tamponade, hemorrhage control and cardiac massage. Concurrent induction of anesthesia and the application of positive pressure ventilation in the setting of tamponade physiology can reduce preload to an extent that may result in profound hemodynamic instability. Resuscitation with blood products must be started (through wide bore peripheral or central venous access, e.g., Cordis line, and often expedited using a massive transfusion protocol) to maintain cardiac filling pressures, and the thoracic operative field must be prepared and surgeon-ready before induction. Penetrating bodies found in situ are generally left in place until the chest is opened, in case of concomitant vascular and solid organ injuries.8,9
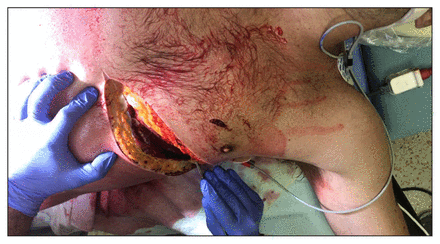
With the arms of the supine patient abducted to 90° on arm boards and the breast retracted cephalad, an incision using a no. 10 scalpel is made from the sternal border of the fourth or fifth intercostal space to the left posterior axillary line along the curve of the rib (Figure 3). The inframammary fold is a reliable visual landmark for this space. The intercostal muscles and pleura are subsequently transected with curved scissors along the superior margin of the rib below. A Finochietto retractor is then placed with the instrument joint on the lateral side of the incision. Before spreading the ribs, large surgical sponges may be used to cover the incised edges to avoid injury from rib spikes. Once opened, the incision can be extended medially for further exposure. To improve exposure and protect the pleural surface during the procedure, ventilation to the left lung can be reduced by temporary right mainstem bronchial intubation. Surgeons must remain vigilant about possible concurrent right thoracic injuries, even as they focus on manoeuvres in the left chest. A LAT can be supplemented by a right-sided chest tube to screen for a right hemopneumothorax that may contribute to hemodynamic instability, which may warrant exploration.
Resuscitative thoracotomy by way of the left anterolateral thoracotomy. The steps in an anterolateral thoracotomy for cardiac trauma include incision (left anterolateral thoracotomy at the fifth intercostal space or inframammary fold); evacuating the hemothorax; opening the pericardium anterior to the phrenic nerve; control of the cardiac hemorrhage (digital control); cross clamping the aorta and initiating cardiac compressions if there is an insufficient response to the above measures (this may require mobilization of the inferior pulmonary ligament); and cross clamping the pulmonary hilum at end expiration to control associated pulmonary injuries.
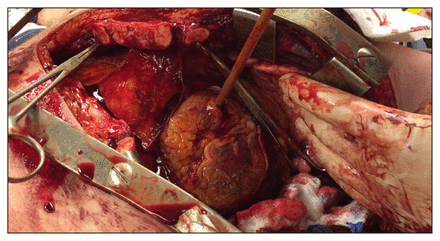
An initial right thoracotomy may be preferred for patients with right-sided chest injuries.10 Otherwise, the LAT incision can be carried into the contralateral thorax as a bilateral anterior thoracotomy (clamshell) incision by cutting the sternum with heavy scissors, a Lebsche knife or a Gigli saw. Using 1–2 retractors, the clamshell incision is opened for further exposure by extending the thoracotomy posterolaterally. In desperate scenarios, a gloved assistant can manually hold the incision open.11 The internal mammary arteries can be identified in a vertical plane about 1 cm from the lateral edges of the sternum (with a variable course). Rarely, they may be ligated as the incision is extended across the sternum. Although these vessels do not often initially bleed owing to vascular spasm, they must eventually be ligated at both the proximal and distal ends before final closure. A LAT is employed as the classic approach to resuscitative thoracotomy, but the clamshell incision provides access and improved visualization in poor lighting to every thoracic structure, except the posterior diaphragm and superior esophagus. A LAT with clamshell extension is often the incision of choice where wide exposures are required for injury control and repair (Figure 4).11–13
An anterolateral thoracotomy can be extended to a clamshell thoracotomy by transection of the sternum with a Lebsche knife. This manoeuvre affords excellent visualization of most intrathoracic structures, especially in low light settings.
Median sternotomy
Patients with injuries to the cardiac box who are hemodynamically stable can be assessed with immediate sternotomy and exploration in the operating room.14 Although sternotomy requires technical precision and attention to detail (i.e., to avoid postoperative complications), it also affords excellent visualization of the anterior heart and great vessels, and therefore enables the deployment of multiple operative techniques. A sandbag positioner can be applied posteriorly between the shoulder blades to better expose the midline (particularly for patients with obesity).15 The suprasternal notch and xiphoid process are first identified to prepare the incision between these 2 points. The initial skin incision is deepened to the sternal bone with cautery, which is then used to trace the midline and divide the interclavicular ligament found at the superior aspect of the manubrium. This prevents subsequent binding and failure of the sternal saw. The jugular venous arch may require ligation or cauterization if closely approximated to the sternal notch. Blunt digital dissection is then engaged to rapidly separate the xiphoid process and manubrium from the underlying mediastinal structures. Opening up the retrosternal space provides additional safety from saw-associated trauma. An osteotomy is generally started from the caudal end, rather than the top, as extra steps may otherwise be needed to cut the sternoclavicular ligaments and develop an adequate retromanubrial space to insert the saw.16 It is critical to keep the saw within the midline of the sternum to avoid shearing into either side of the chest. This is particularly important in the lower sternum as it is thinner and more vulnerable to saw deviation.17 If an electric or pneumatic saw is unavailable, a large straight bone cutter can be applied upward from the xiphisternum instead.18 The anesthesiologist ideally holds patient ventilation, and the osteotomy proceeds with the saw angled upwards to avoid any injury to the underlying pleura and mediastinum. With towels and sponges covering the cut sternal edges to control bleeding, the retractor is then placed into the sternum. The retractor blades should ideally contact the distal manubrium to minimize any additional fractures upon rapid thoracic distraction.17 The mediastinal fat can be dissected, and pleurae can be pushed aside. It should be noted, that in general, a median sternotomy should be resevered only for patients with anterior thoracic stab wounds. Procedures to the posterior heart can be particularly challenging to do with efficacy through this incision; therefore, a bilateral thoracotomy is preferred for all gunshot wounds and most other penetrating injuries, particularly outside of the cardiac box.
Pericardiotomy
After the sternal halves are retracted, the pericardium is grasped between 2 mosquito forceps (or Allis clamps) and a small incision is created with a no. 10 scalpel blade along the midline. Forceps will be unhelpful in the context of a tight, fluid-filled pericardial space. Damage to the underlying epicardium can be avoided by simply maintaining the blade at an oblique angle. The resulting defect is extended longitudinally with Metzenbaum scissors and T extensions are created along the aortic and diaphragmatic reflections. Cautery can also be used to open the pericardium as long as care is taken to avoid direct application to the myocardium, which can start rapid wide-complex tachyarrhythmias (i.e., ventricular tachycardia or fibrillation). Likewise, the thymic tissue can be divided with cautery or pushed away to expose the pericardium covering the ascending aorta. Access to the heart must be large enough to allow the insertion of 2 hands to perform internal cardiac massage when indicated. A simple pericardial sling is created by tautly suturing the open edges to the skin or wound towels, and therefore preventing retraction from dehydration.6 The hemopericardium should be evacuated, and the cardiac rhythm noted for potential cardiac massage or defibrillation. Attention to a sudden change in arterial pressure upon opening of the pericardium (in the presence of a tamponade) is essential, as there will be an initial rise in the arterial pressure. If a continuous intrapericardial bleed is present, this rise will be followed by a drop in arterial pressure owing to the continuous blood loss.
In open cardiac massage, the heart is squeezed between 2 flat palms from the apex, avoiding any digital penetration into the myocardium. During compressions, the fullness of the heart can provide a sense of the patient’s volume status and the adequacy of the resuscitation. The effectiveness of compressions can be gauged by arterial line waveforms or by end-tidal carbon dioxide measurements, when these adjuncts are available. When needed for ventricular fibrillation, defibrillation by way of internal paddles commences at 10 J and is repeated at 10–50 J, as required.
From a left anterolateral thoracotomy, the pericardium is elevated and incised with a blade precisely 1–2 cm anterior to the phrenic nerve. The incision is then extended parallel to the nerve with scissors. The phrenic nerve lies on the pericardial surface, and is immediately anterior to the pulmonary hilum. Care should be taken to avoid damaging the phrenic nerve by dividing it, or by cutting the pericardium too closely and causing a retraction injury to the nerve. After releasing a cardiac tamponade, open cardiac massage can begin against the sternum with 1 palm on the posterior aspect of the heart.19
It should be noted that when a thoracotomy is performed for trauma, the pericardium must always be opened. External inspection of the pericardium is not sensitive for intrapericardial blood, even in the presence of tamponade.
Cardiac hemorrhage control
A diverse set of techniques for attaining rapid cardiac hemostasis is a critical asset in damage control and emergency trauma surgery. After inspection of the cardiac surface for any wounds, immediate hemostasis by digital pressure may be adequate to proceed to definitive repair (being very careful to not increase the size of the wound). When faced with multiple cardiac lacerations, stapling (6 mm skin staples [Auto Suture 35 W, United States Surgical Corporation]) can also be employed for temporary bleeding control. Although some clinicians reinforce the stapled closure with sutures, staples can be left in place without reinforcement when necessary or preferred. Unfortunately, some injuries, such as largecaliber gunshot wounds or injuries near the coronary arteries, cannot be appropriately managed by way of cardiac stapling.20 Balloon occlusion with a clamped Foley catheter or cuffed endotracheal tube may address larger defects by inflating the balloon with saline inside the chamber and gently withdrawing it against the wall. Excessive traction can enlarge the laceration further and create a fatal disaster. Users must be extremely cautious not to inadvertently pull the balloon catheter out and thereby enlarge the laceration into a nonrecoverable scenario. With the balloon inflated and extremely gentle traction applied to the catheter, Teflon-pledgeted sutures can then be passed through the ventricle from side to side over the balloon. The thin wall of the right ventricle puts the inflated balloon at risk of puncture as each suture is placed. Pushing the catheter and balloon into the ventricle with each bite of the suture will mitigate this complication, although blood loss may be severe. An alternative option is to employ a cuffed endotracheal tube. This provides the advantage of increased manual stability while sewing. However, excessive traction on either device can enlarge the initial laceration and lead to death. Conveniently, direct venous access may be obtained through the Foley catheter itself for medication boluses (i.e., connect intravenous fluid tubing). A novel hemostatic vacuum device, which consists of a central pillar that occludes the wound by way of peripheral suction, has also been employed to obtain rapid hemostasis, and therefore allow the surgeon to address synchronous injuries.21,22 Atrial bleeding is fairly easy to control with a Satinsky vascular clamp, followed by sutured repair or stapled resection (linear stapler with a vascular load).
Temporary inflow occlusion with vascular tapes or atraumatic clamps applied to the intrapericardial superior vena cava and inferior vena cava may be necessary to visualize and control extensive or high-pressure cardiac wounds.23,24 With a longitudinal perforation or considerable rupture of a ventricle, the technique of inflow occlusion is useful in avoiding cardiopulmonary bypass (CPB). Patients will immediately become hypotensive when the vena cava are occluded. Curved aortic or angled vascular clamps are first applied to the superior and inferior vena cava. The inferior vena cava can be accessed either within the pericardium or between the liver and diaphragm. As the heartbeat slows, horizontal mattress sutures are inserted rapidly on either side of the defect and then crossed to control hemorrhage. A continuous suture is placed to close the defect and before it is tied down, air is vented out of the elevated ventricle by releasing the clamps on the vena cava. This cardiac response also occurs with compression of the right ventricle and pulmonary artery. Internal paddles and other resuscitation tools should be readily available. This technique must be limited to short intervals of occlusion with repeated relief, or successful rhythm restoration is unlikely after about 3 minutes.25 For injuries to a more vulnerable or friable myocardium, manually compressing the right atrium will result in the partial inflow occlusion necessary to repair the ventricle.19 Injuries involving the lateral wall of the left ventricle, left pulmonary veins, left atrial appendage or the left pulmonary artery are accessed through a cupping manoeuvre to lift the ventricles out from the pericardial well. This should be done fairly slowly by running the fingers of the right hand between the diaphragm and the right ventricle, and then sweeping them posteriorly and cephalad. The hand cups the apex of the left ventricle, which is subsequently elevated anteriorly out of the pericardial well. This nuanced sequence will avoid rapid subsequent hypotension. Meanwhile, placing several pericardial retraction sutures in the posterior part of the pericardium is also helpful to maximize exposure. Caution in technique is essential if cardiac massage is required to promote prograde perfusion. An open hand methodology prevents punctures of the heart.
It should be noted that as procedures such as inflow occlusion are considered or engaged, additional (and early) consultation with a perfusionist (i.e., heart-lung machine) and cardiac surgeons becomes increasingly important. Scenarios such as ventricular septal punctures or acquired ventricular septal defects are nuanced and mandate bypass.
Aortic clamping
In general, aortic cross clamping can worsen hemorrhage above the diaphragm. However, when a patient is close to exsanguination, occluding the descending thoracic aorta may be necessary to redistribute any remaining aortic pressure to the brain and myocardium, and may improve myocardial contractility and stroke volume. From an anterolateral thoracotomy, the left lung is elevated anteriorly, followed by an incision to the mediastinal pleura and the inferior pulmonary ligament. The aorta can be identified just above the diaphragm as the first tubular structure anterior to the thoracic spine. Blunt dissection is performed to separate the pleura along the anterior and posterior borders of the aorta. This must be just enough to place a clamp without severely disrupting the thoracic and spinal blood supply. Perfusion to the spinal cord can also be maximized if the clamp can be placed closer to the aortic hiatus of the diaphragm. Manual occlusion between the thumb and index finger, or simply against the vertebral body as a desperate measure, can be engaged before formal clamping. To avoid esophageal perforation, an in situ nasogastric tube may be used as a guide to differentiate the aorta from the esophagus.
Cardiac repairs
Once temporary hemostasis is achieved (often with a delicate single finger), patients with signs of life should proceed to the operating room for definitive repair. Optimization of technical conditions (e.g., lighting, field organization, operative exposure, instrumentation, suture availability) are essential, both to avoid iatrogenic injury and to create a precise and enduring repair. The specific reconstruction technique depends on the characteristics of the injury, the resources available in the resuscitation area or operating room, as well as the operator’s experience and preference.
After pericardiotomy, the heart produces an additional lateral rocking motion without the pericardium holding it in place. This movement can be safely minimized by an assistant’s Satinsky clamp on the acute anteroinferior angle of the right ventricle.26 This technique is often more straightforward in a heart that is less filled with blood. Use of the Octopus tissue stabilizer (Medtronic) is also a reasonable alternative, if available. Simple ventricular laceration repair involves passing double armed 4–0 SH or 3–0 MH polypropylene sutures under the digital occlusion and out the other side in 1 pass. The 2 ends of the sutures are gently pulled to approximate the lacerated edges from bleeding, and the needle is reinserted across the finger and back out the other side. This completes a figure-of-8 stitch as the finger is subsequently withdrawn. These steps are repeated along the defect as needed. A potentially safer alternative is to employ pledgeted polypropylene sutures with a horizontal mattress technique, when possible, to reduce the risk of tearing the heart tissue. Although Teflon pledgets are sometimes unnecessary on a thick and robust myocardium, they can be helpful for a friable and edematous heart, the right ventricle or areas with surrounding contusion and hemorrhage. This technique generally provides an additional seal and protection.19,27,28
It is important to highlight that the principles of suturing cardiac muscle are similar to sewing other soft structures such as the liver and pancreas. More specifically, correctly selecting the optimal suture and needle type and size, maximizing delicate soft tissue handling, using the entire curve of the needle for insertion and egress, tying flat smooth knots and avoiding all regional distractions are critical to technical success.
A vigorously pumping heart can create difficulty in passing the needle through both edges of the wound within 1 movement. Instead, an additional needle holder in the nondominant hand can also be used to catch the needle from inside the defect after it is inserted. The needle is then passed through the opposite edge of the laceration. Timing the needle entry to diastole can also prevent inadvertent slashing of the cardiac musculature. Furthermore, if a Foley catheter is employed to control the bleeding, the catheter can be carefully pushed into the chamber each time the needle is inserted, thereby preventing perforation of the balloon. Larger defects, including gunshot wounds, may be closed with interrupted horizontal mattress sutures instead.6 Whichever strategy is employed, adequate suture bites through the myocardium must be ensured to lower the risk of tissue tearing. This is particularly important for the thinner right ventricle. As previously noted, the selection of needle size is critical to success.
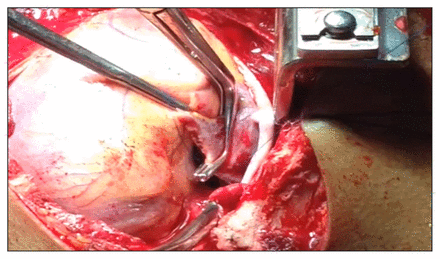
Atrial defects are repaired by placing a vascular clamp under the perforation (Figure 5). Preventing additional traction to the atrial wall is essential to avoid lacerating it. Simple, continuous stitches with 5–0 polypropylene sutures on an RB needle can be used. Alternatively, a 6–0 polypropylene suture may be employed if the atrial tissue is exceptionally thin. Running horizontal mattress stitches may be more appropriate for thin atrial walls, which require a technique that spreads tension along the entire wound edge.6 When the injury cannot be controlled with a single clamp, multiple Allis clamps can be engaged in a row to pinch the wound edges together, followed by mattress sutures underneath. If the atrium is especially dilated, pledget reinforcement may be required. When time is limited, or such bioprosthetic materials are not readily available, small pieces of the pericardium can also be used to buttress sutures.29 Two needles from a double-ended suture are passed through the pericardial sling on 1 side, then across the laceration, and out the opposite pericardial edge. Pledgets are cut and fashioned into a particular size and the 2 ends are pulled. The second pledget is apposed to the ventricular wound by irrigation, and then the sutures are tied to complete the stitch. This simple technique is also useful when small pledgets are required for vascular anastomoses and repairs (Figure 6).
Cardiac repair. Ventricular injury (shown from below). Ventricular injuries are often immediately controllable with digital pressure. Lacerations can be rapidly approximated in damage control situations with skin staples. Definitive repairs are often done with simple or horizontal mattress interrupted sutures, using 2-0 or 3-0 polypropylene sutures on MH-tapered 3/8 circumference needles.
Cardiac repair. Right atrial repair (shown from above). The atria are thin-walled structures that, when inured, can be grasped, approximated and elevated with Satinsky clamps. Interrupted sutures can be supplemented with pericardial or Teflon pledgets if a risk of suture pull-through is perceived.
As mentioned, the beating heart often presents a challenge for accurate suture placement, posing a risk of needle-stick injury during digital occlusion. Intravenous administration of adenosine has therefore been employed to induce a brief asystole and thereby facilitate repairs on the stationary heart.30–32 Low doses of adenosine (3–12 mg) stop the heart for 10–30 seconds, during which repair and comprehensive inspection are completed. Adverse effects, including atrioventricular block and hypotension, usually resolve when the drug is stopped, making adenosine a reliable adjunct to repair.30
Alongside adenosine infusion, several additional manoeuvres for the inspection and repair of challenging cardiac injuries are relevant. Management of wounds to the posterior aspect of the heart require special care, as lifting the heart kinks the great vessels, causing bradycardia, hypotension and cardiac arrest. However, to access the posterior of the heart, it must often be “flipped up” before suture repair. Close communication with the anesthesiologist and rapid surgical technique are essential, given the typical induction of complete cardiac arrest after positioning. As a result, intermittent restoration of the heart back into its natural position is required for cardiac relief during prolonged repairs. Alternatively, gentle lifting of the heart by gradually stacking 1–3 folded laparotomy pads provides time for the heart to adapt to the planned displacement. Depending on availability, off-pump cardiac stabilization devices are also an option to gain safe elevation and rotation for cardiac exposure.33 In desperate cases, it may be necessary to elevate an atraumatic clamp applied to the acute anterior–inferior margin of the right ventricle and repair the wound as quickly as possible.26
Defects adjacent to the coronary arteries also warrant additional comment as coronary blood flow can be inadvertently compromised during the repair. Interrupted, horizontal mattress sutures are placed beneath the bed of the coronary vessel to prevent vascular constriction. Pledgets may be omitted, unless the sutures are likely to tear through the myocardium and vessel. Suturing alongside a coronary artery is guided by monitoring for ST segment changes or Q waves. If these occur, urgent stitch removal and resuturing may be required.19
Despite the multiple strategies that augment cardiorrhaphy, injuries adjacent to the coronary arteries may require a sutureless approach. Application of a collagen mesh dressing covered by fibrin glue to occlude a stab wound near a branch of the circumflex has previously been reported.34 Likewise, defects that are complex, such as large lacerations or a coronary sinus injury, may necessitate the use of autologous pericardial or synthetic patches, which are subsequently strengthened by applying biological glue agents.14,35 When neither are available, tissue patches can be obtained from the anterior rectus fascia. Institution of CPB when bleeding is impossible to control may facilitate patch grafting and further reinforcement using an omental or muscle flap.36
Foreign body removal
Occasionally, trauma surgeons may encounter an in situ cardiac foreign body. Symptoms attributable to these foreign bodies, including cardiac tamponade and arrhythmia, are indications for removal.37,38 However, simple extraction of the foreign body does pose further risks of damage to a patient with potential instability (e.g., a projectile that is near a coronary artery or is deeply embedded within the myocardium and tamponading the wound).39,40 Manipulation of foreign bodies contained within the left side of the heart requires great care and speed owing to the high risk of critical embolization.41
When removal is indicated, embedded projectiles can be manually extracted with forceps after sewing pledgeted, double-armed, horizontal mattress sutures around the body and slowly tightening the stitches during extraction.33 Nails must be removed by careful twisting instead of simply pulling and risking damage to the surrounding wound edges. Alternatively, purse-string sutures may be placed at the entry site to correct the defect immediately after removal. Intravenous adenosine infusion can also be considered as an adjunctive manoeuvre to lower contractility and facilitate safe extraction of the foreign body.40 The concomitant use of intraoperative transesophageal echocardiography may also be particularly helpful for visualization of a bleeding heart, reinforcing assessment of the foreign body and guiding surgical instruments.42 The decision to institute CPB must be balanced against its risks to the patient, but when appropriate, should be triggered early in the surgical process given its clear benefit of ensuring adequate repair or foreign body extraction.
Coronary artery injuries and cardiopulmonary bypass
Injuries to the coronary arteries are infrequent and associated with high rates of prehospital and inpatient mortality.43,44 Decision-making and treatment (including the decision to go on CPB can be complex and require early collaboration with a cardiac surgeon, if possible. This close relationship with cardiac surgeons is essential as their use of CPB may be more liberal and allow avoidance of prolonged inflow occlusion. The general approach to lacerated coronary arteries consists of ligating injuries to small branches or distal vessels (< 1 mm in diameter) and bypassing major arteries in patients with proximal coronary arterial wounds, although small puncture injuries can be repaired with 6–0 or 7–0 polypropylene sutures.
Ligation of a distal or narrow artery must be followed by a period of close observation for possible cardiac ischemia or failure. Injuries to the left anterior descending artery, which are relatively common, are particularly prone to these complications, as they can devascularize up to 50% of the left ventricle. If a considerable myocardial injury is identified early enough, ligation should be reversed immediately. Intraluminal shunts that bridge the lacerated ends of the vessel have also been used to stop bleeding while conserving regional ventricular function and perfusion distal to the laceration.45,46 Using Potts scissors, the wound can also be carefully extended to facilitate insertion of the shunt and subsequent distal anastomosis.
Coronary artery bypass may be the only approach available to salvage a lacerated vessel, even in patients with trauma with major comorbidities and substantial risks consequent to bypass itself. Off-pump coronary artery bypass grafting (CABG) may achieve repairs with less anticoagulation, without cardioplegic arrest and without the risks associated with CPB in patients who are hemodynamically stable. When heart-stabilizing devices are not immediately available for off-pump CABG, the centre of a Teflon patch may be cut to form a square frame that encloses the anastomotic site.47 The corners of the patch are deeply sutured into uninjured myocardium, and gentle upward traction is applied to the loosely tied threads. This locally immobilizes the target and prepares the anastomotic site. Bypass can then proceed with a single 6–0 or 7–0 polypropylene suture on a fine taper–point needle. The left internal mammary artery is often considered the first choice for aortocoronary bypass grafting, despite its poor patency rates during episodes of vascular spasm in a patient in an unstable condition.48 Use of this vessel is also limited when CPB is instituted through a clamshell incision that transects the artery. Alternatively, reversed saphenous vein grafting remains an option.
Injuries causing substantial cardiac dysfunction, arrhythmias or impending intractable heart failure despite attempts at repair may necessitate engaging acute CPB. Other indications for CPB include the inability to manage a wound owing to its large size or location, as well as failure to repair the wound despite hemodynamic stability or inotrope administration.49 In these cases, CPB provides a platform for thorough inspection and exposure of the heart, as well as definitive myocardial repair within a bloodless field. Uncontrollable bleeding or postoperative coagulopathy and inflammation from systemic heparinization during CPB, especially in patients with multiple injuries, can be obviated to some extent with heparin-bonded circuits.43 Marginal cardiac dysfunction, such as that resulting from distal vessel ligation, may be adequately treated with an intra-aortic balloon pump to provide sufficient cardiac output.
Extracorporeal life support
Extracorporeal life support (ECLS) is emerging as an adjunct in the care of patients with penetrating chest trauma and refractory shock. Concerns about systemic anticoagulation had previously limited ECLS use in trauma, but have been offset by potential advantages, including rapid cannulation, and the well-documented feasibility of avoidance of therapeutic anticoagulation when heparin-bonded circuits are used.50 Cardiopulmonary failure is managed using veno arterial ECLS with femoral–femoral cannulation (i.e., percutaneous access with a Seldinger technique), preferably without a cutdown incision, to prevent further bleeding. The access cannula is positioned near the junction between the right atrium and the inferior vena cava to optimize venous drainage. The return cannula is directed toward the distal aorta, offering complete circulatory and respiratory support. Extracorporeal life support enables trauma teams to control temperature at around 36°C, which may be useful in helping to reduce secondary brain injury for patients with cerebral injuries or those who have received cardiopulmonary resuscitation. In cases where a complex cardiac operation cannot be tolerated in a patient who has been resuscitated or where myocardial stunning results in transient cardiogenic shock, ECLS may serve as a bridge to recovery, or to further investigations and interventions.
Operative closures
Pericardial closure
Pericardial closure is favoured in most nontrauma operations to minimize postoperative retrosternal adhesions (post sternotomy) and prevent lateral cardiac herniation (post LAT). This is especially true in cases of a repeated sternotomy, as it improves hemodynamics and protects against cardiac tamponade. However, in patients with trauma, closing the pericardium has the potential to lead to iatrogenic tamponade because of myocardial edema due to direct injury or resuscitation. The risks of sealing the heart in these cases may outweigh the benefits. When primary closure is feasible, it can be performed by approximating the edges of the pericardium with a 2–0 absorbable (Vicryl) continuous stitch at 1 cm intervals beginning at the cranial end. If a reoperation is possible or intended, nonabsorbable sutures may be employed to guide future reopening. A 2 cm gap is left at the diaphragmatic end for a mediastinal drain to be placed anterior to the defect. When closure is still preferred despite cardiac dilation or despite a limited supply of native pericardial tissue, the defect can be conveniently covered with pericardial fat pads that are readily dissected and sutured onto the pericardial edges.
Drains
Proper placement of mediastinal and pleural tubes can prevent complications from recurrent hemopneumothoraces, cardiac tamponade or infection. Prophylactic antibiotics are also justified for thoracostomy tubes in patients with penetrating injuries.51 Standard 24–32 French chest tubes are inserted through the intercostal spaces in the midaxillary line. Although the fourth or fifth intercostal spaces are often used, they may not be available if a thoracotomy was performed at the same level. Drains can alternatively be placed through the lower intercostal spaces with visual, manual or sonographic guidance. Air drainage is best achieved by placing the drains in an anterior direction, whereas the tube can be directed posteriorly for evacuating blood. Alternatively, tube thoracostomy can proceed through epigastric incisions by ensuring they are placed laterally within the rectus fascia to prevent drain site hernias.52 Mediastinal or pericardial drains can be inserted along the midline, often below the median sternotomy incision in the epigastrium (angled tubes may be particularly helpful). As previously discussed, a distal gap is left behind when closing the pericardium to facilitate drainage through the tip of the pericardial drain. Drains are ideally secured to the skin with 0 or no. 1 Ethibond sutures. It is critical to carefully label all drains (i.e., pleural space or mediastinum or pericardial) within the thorax in the postoperative setting. Negative pressure should not be placed on pericardial drains to avoid compromising fresh cardiac repairs.
Median sternotomy closure
Accurate sternal reapproximation and closure are key factors in preventing postoperative pain, sternal dehiscence and infection.53 Figure-of-8 wires are often used as a fast and stable closure technique that has comparable wound complication rates to new sternal closure methods.53,54 Four to 8 stainless steel wires are passed through the manubrium and body, including one that bridges the manubriosternal joint. Before closure, a towel can be placed between the sternal halves to protect the heart. Minimal bleeding occurs when passing the needle perpendicularly through the bone, employing the needle holder between the proximal and middle-third of the needle and advancing it vertically.9,52 A concave instrument may also be positioned under the sternum to further protect the mediastinum from injury by the needle. Optimal approximation and stability are achieved by inserting wires at equal distances from the midline at the appropriate vertical level. When this is difficult, it may be better to apply the wires around the sternal body between the intercostal spaces. If this technique is preferred, extra caution is essential to avoid damaging the internal mammary arteries and causing a subsequent hemothorax. Once the wires are in place and locked with needle drivers, the towel is gently removed and the mediastinum is rinsed with saline. Definitive hemostasis from the sternal edges is achieved with electrocautery or bone wax. The wires are crossed and lifted upwards (i.e., sternal halves are approximated), and then loosely twisted and cut. The surgical assistant can facilitate approximation by lifting the pectoral girdle forward with their palms on the scapulae. The cut ends of the wire are tightened until the sternal edges come into contact and the wire stumps are buried entirely into the presternal tissue. Internal sternal fixation with absorbable sternal pins can provide additional stability with the possibility of easy re-entry.55 Alternatively, sternal wires can be placed in a simple interrupted manner, spaced 1–2 cm apart. They are then straightened before crossing in a smooth fashion to ensure adequate sternal apposition once twisted.
Delayed pericardial and sternal closure may be necessary if the heart enlarges from primary edema or excessive fluid administration. A temporary thoracic closure may also be required if hemodynamic compromise ensues with attempts at reapproximation. Diuresis with furosemide is a viable option if the patient’s hemodynamics will allow. Otherwise, an abdominal-type plastic covering with sterile surgical drapes, or a genitourinary irrigation bag sewn on to the skin, can be temporarily employed until definitive closing is possible. If the sternum is left open, the patient needs to remain sedated and paralyzed, as coughing or straining can cause cardiac lacerations due to contact of the cut sternal edges with the right ventricle (Hanuman syndrome). It may also be possible to protect against this occurrence by using a vacuum-assisted closure dressing with concurrent placement of a large pad between the sternal edges. Vacuum-assisted closure dressings can also be employed as an alternative to sterile draping with no apparent negative impact on cardiac and respiratory dynamics.56 In extreme cases, where any contact between the heart and the sternal edges compromises cardiac function, sternal stenting is necessary. Two semi rigid chest tubes or twisted wires can be bridged across the mediastinum and sutured against the sternal edges as a quick and simple approach to prevent an edematous heart from compression.57,58
Clamshell closure
Bilateral transverse thoracosternotomies can be closed by 1–2 figure-of-8 stainless steel wires that go through and cross-bridge both parts of the separated sternum. Conventional uncrossed loops into the bone do not prevent anteroposterior displacement of the sternal segments and may pose a risk to the already injured heart.59 The transected internal mammary arteries must also be ligated before closure. The thoracotomy incisions are closed with heavy, interrupted sutures of no. 2 vicryl spanning the ribs above and below the incision (meticulously avoiding the neurovascular bundles immediately below each rib), placed 2–3 cm apart, and tied after all sutures are placed. This is followed by layered re-approximation of the serratus anterior muscle and the subcutaneous and subdermal layers with running sutures of 0, 2–0, and 3–0 vicryl, respectively. The skin is closed with staples. As noted for median sternotomy, if damage control methods are being applied, a temporary, vacuum-assisted closure technique can be used (in conjunction with appropriate placement of drains) to seal thoracotomy incisions until coagulopathy, acidosis and hypothermia are reversed, edema reduced and definitive closure can be achieved.
Skin closure
Skin closure with sutures (as opposed to staples) may allow more rapid re-entry to the chest if a high likelihood of reoperation is predicted.
Postoperative care and pitfalls
Close postoperative evaluation is crucial to reduce the incidence of posttraumatic cardiac sequelae in patients with penetrating cardiac injuries. Postoperative care should include monitoring by electrocardiography and the liberal use of 2-dimensional Doppler echocardiography. Tang and colleagues60 reported abnormal echocardiograms in 17.4 % of patients with penetrating cardiac trauma, with pericardial effusion (47 %) being the most common finding (followed by wall motion abnormalities and reduced ejection fraction). Further investigation for causes of postoperative cardiac failure may elucidate coagulopathic tamponade, hemorrhage from the repair site or acute myocardial infarction. Heart failure typically requires inotropic medications and, occasionally, electromechanical device assists for cardiac support. Accordingly, continuous hemodynamic monitoring is essential. Unexplained hypotension could be the result of bleeding from a transected internal mammary artery. This iatrogenic complication should be prevented by active search and early ligation during the initial resuscitative procedure, and should be aggressively pursued and controlled with early re-exploration in the postoperative period.
Posttraumatic acute myocardial infarction can be diagnosed with a combination of segmental movement disorders in transesophageal echocardiography, electrocardiogram abnormalities, and serum tropinin I levels. However, electrocardiogram abnormalities and serum tropinin I levels are insufficient alone and require further confirmation by another method, as surgical and resuscitative manoeuvres themselves create changes in both.61 Subsequent cardiac assessment should incorporate differentiation of hemorrhagic, dynamic or stenotic causes of infarction. Complete heart block and other conduction system abnormalities, which have been reported to occur in 2.8 % of patients with penetrating cardiac injuries, may warrant temporary placement of epicardial wires or transvenous pacing.62 Findings, such as exertional dyspnea or new murmurs, may suggest an intra-cardiac injury such as a ventricular septal defect, which may require transcatheter closure, depending on the magnitude of associated symptoms. Similarly, other complex cardiac sequelae such as valvular injuries require close multidisciplinary communication and teamwork. Synchronous valvular injury in particular must be ruled out in cases of penetrating cardiac injuries, as 3%–8 % of patients will have a concurrent trauma to 1 or more heart valves.
Despite modern techniques and standard hygiene within cardiac surgery, sternal wound infections still occur at relevant rates, with associated in-hospital mortality rates of up to 35%.52 In a patient with trauma, who may have undergone a rapid sternotomy with less-thansterile technique in a rushed envrionment, special attention must be paid to postoperative infections. Although antimicrobial prophylaxis has been suggested for cardiac surgery, controversy remains over optimal dosing, duration and timing. Antimicrobial prophylaxis or treatment for other complications, such as empyema or sepsis, should be based on factors such as the hospital antibiogram and specific sites of infection. A combination of medical treatment and irrigation, vacuum-assisted closure or flap coverage should be used for these infections if they arise.
Conclusion
Penetrating cardiac injuries pose complex strategic, technical and logistical challenges that test the performance of entire trauma systems. Acute care surgeons, with training and experience in the decision-making and operative aspects of penetrating cardiac injuries, and with knowledge of systems of acute care, are well positioned to lead comprehensive resuscitative and operative efforts. Technical depth and agility with respect to damage control physiology and resuscitation, surgical exposure, injury control, cardiac repair and chest closure can reduce the downstream consequences of penetrating cardiac injuries and the complications of surgery. With preparation, trauma and acute care surgeons can streamline the response to one of the most acute, time-dependent and complex surgical crises. Early collaboration with cardiac surgeons and their perfusionist teams, when available, can also be life saving.
Acknowledgement
The authors are grateful to Dr. David Evans and Trauma Services at the Vancouver General Hospital for conceptual support and images included in this article.
Footnotes
Competing interests: C.G. Ball is coeditor-in-chief of CJS; he was not involved in the review or decision to accept this manuscript for publication. No other competing interests were declared.
Contributors: All authors (A. Lee, M. Kaminsky C.G. Ball and S.M. Hameed) were involved in the literature review, and the design and writing of the article. All authors participated in final edits and approved the final version for publication.
- Accepted December 14, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/