Abstract
Background: Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) has recently shown promise for the treatment of patients with various types of peritoneal carcinomatosis (PC). However, it is an extensive procedure that is associated with a variety of morbidities. We evaluated the safety and clinical outcomes of CRS-HIPEC performed at our centre.
Methods: Patients with abdominal malignancies who underwent CRS-HIPEC between February 2005 and December 2018 at the Centre hospitalier de l’Université de Montréal (CHUM) were retrospectively reviewed.
Results: A total of 141 patients were identified (66 with appendiceal cancer, 62 with colorectal cancer, 10 with mesothelioma and 3 with small intestinal tumours). The median age was 55 years. Median overall survival (OS) was not reached for patients with appendiceal tumours; it was 38.3 months for colorectal cancers. Among patients with colorectal cancer, survival was significantly better for those who received intraperitoneal HIPEC with oxaliplatin (74.9 mo) compared with mitomycin C (29.1 mo) (p = 0.006). Complete cytoreductive surgery and low peritoneal carcinomatosis index were associated with the highest overall survival in patients with appendiceal tumours and those with colorectal tumours.
Conclusion: CRS-HIPEC can be performed with acceptable morbidity in patients with PC. These results validate the outcomes of previously reported trials, but further prospective trials are warranted to determine which patients will most benefit from the addition of HIPEC to CRS.
Colorectal cancer (CRC) is the third most common cancer worldwide.1 Peritoneal carcinomatosis (PC), defined as the spread of tumours over the peritoneal surface lining of the abdomen, is the second most common site of recurrence in patients with CRC and accounts for up to 25%–30% of all recurrent or metastatic CRC.2 In up to 25% of cases, PC is the sole site of metastasis.3
Patients who develop PC have a poor prognosis. For example, with systemic therapy the median survival of patients with PC who have CRC primaries is up to only 15 months.4,5 With extensive research and technical advances, cytoreductive surgery (CRS) in combination with perioperative systemic therapy and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) showed promising results and improved survival.6,7 Verwaal and colleagues, in a randomized controlled trial, showed that CRS-HIPEC significantly increased overall survival to 22.3 months compared with 12.6 months with standard palliative chemotherapy with 5-fluorouracil (5-FU), which is now an outdated therapy.8 On the other hand, there was no survival gain when HIPEC was added to CRS and systemic chemotherapy in the PRODIGE 7 trial.9,10 Thus, it is evident that CRS has a benefit in PC of CRC, but current evidence with a proper randomized controlled trial is lacking for the use of CRS-HIPEC as the standard of care. In addition, the selection criteria for patients who would benefit from the addition of HIPEC to CRS have yet to be determined in CRC. However, it is generally agreed that patients with better prognostic factors would benefit the most. These factors include low peritoneal cancer index (PCI) score, no or low liver disease burden, low patient comorbidity profile and longer disease-free survival (DFS) in metachronous carcinomatosis.11
Appendiceal mucinous tumours are rare, accounting for only 1% of all cancers.12 Mucinous neoplasms of the appendix constitute a heterogeneous group of neoplasms, ranging from adenomas to mucinous adenocarcinomas.13 To simplify this wide spectrum of disease, these tumours are classified as low and high grade.14 Others have classified these tumours as disseminated peritoneal adenomucinosis (DPAM) and peritoneal mucinous carcinomatosis (PMCA).15 In pseudomyxoma peritonei of appendiceal origin and peritoneal mesothelioma, CRS-HIPEC is considered the standard of care as it can clearly prolong DFS and overall survival (OS).16–18
CRS-HIPEC is a challenging procedure offered only in high-volume centres; it requires experienced surgical teams and substantial infrastructure. Even in these settings, it is associated with morbidity. Given that it is a new treatment approach, not much data have been published to date on outcomes in the Canadian population. Thus, we aimed in this study to establish that CRS-HIPEC is a feasible and safe procedure. We also aimed to evaluate our own experience regarding morbidities, complications and survival outcomes in real-life practice.
Methods
This was a retrospective analysis approved by the research ethics board of the Centre hospitalier de l’Université de Montréal. All patients with abdominal neoplasms with peritoneal involvement who underwent CRS-HIPEC between February 2005 and December 2018 at our centre were recorded. Patients with appendiceal tumours, mesotheliomas and PC secondary to colorectal and small bowel adenocarcinomas were included. Patients with ovarian and gynecologic neoplasia were excluded. Data on age, date of diagnosis, synchronous or metachronous tumours, body surface area, perioperative chemotherapy, pathology, postoperative complications, duration of intensive care unit stay, duration of total hospital stay, transfusions and dates of recurrence and death were collected. Our study focused on appendiceal and colorectal cancers; thus, mesotheliomas and small bowel tumours were excluded from the survival analysis.
Eligible patients had cytoreductive surgery as first described by Sugarbaker.7 After complete cytoreduction, HIPEC was administered. In most cases, patients received either mitomycin C over 90 minutes or oxaliplatin over 30 minutes, after the drug was heated to 40°C–42°C. The chemotherapy agent was selected according to the type of disease, the patient’s previous exposure to chemotherapy and the performance status of the patient.
The PCI score was determined intraoperatively by the surgeon, along with the completeness of cytoreduction (CC) score. CC-0 indicates that no macroscopic residual cancer remained, CC-1 indicates that no nodule larger than 2.5 mm in diameter remained and CC-2 indicates that nodules between 2.5 mm and 2.5 cm in diameter remained.19 PCI scores range from 1 to 39.19 Postoperative complications were recorded according to the common terminology criteria for adverse events, version 5.20 Comprehensive Complication Index (CCI) score was calculated using a downloaded calculator. This recently studied index is highly sensitive as it represents the sum of all complications, each weighted by its severity.21
Descriptive statistics were used. We calculated p values for differences between types of intra-abdominal chemotherapy using χ2 tests. DFS, progression-free survival (PFS) and overall survival (OS) were analyzed using Kaplan–Meier curves plotted with GraphPad Prism 7. Statistical significance was defined as p < 0.05, obtained by a 2-tailed test.
Results
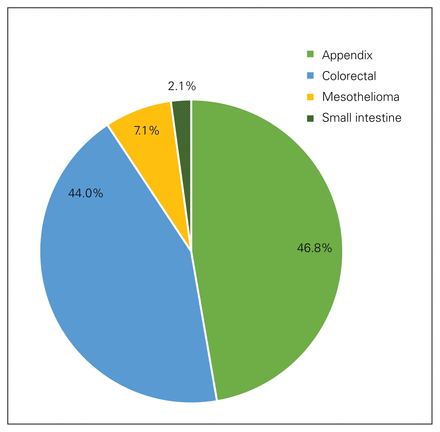
Of the 141 patients who underwent CRS-HIPEC between February 2005 and December 2018, 58 were male and 83 were female, with a median age of 55 years (range 15–77 yr). In total, 46.8% (66 patients) of the tumours were neoplasms of the appendix and 44.0% (62 patients) were colorectal cancers (Figure 1). In appendiceal tumours, 39.4% (26/66) of the cases were DPAM while 56.1% (37/66) were PMCA. Excluding those with mesothelioma, 90 patients (68.7%) had synchronous disease.
Distribution of primary neoplasms in 141 patients who underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Surgical outcomes
At the time of cytoreductive surgery, 22.0% of patients underwent splenectomies and 23.4% had resection of liver metastasis, with a mean of 1 bowel anastomosis per operation (Table 1). Blood transfusion was required in 36 cases with an average hospital admission of 16.75 days, including stays in the intensive care unit if required. The most common complications were gastrointestinal, including ileus, and the mean CCI score was 17.7. No 30-day mortality was observed. Two patients experienced anastomotic leak. Complete cytoreduction (CC-0) was achieved in 89 patients (63.1%), with an average PCI score of 15.
Clinical and operative characteristics of patients who underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
Chemotherapy
Systemic neoadjuvant chemotherapy was administered to 77 of the 141 patients (54.6%). Neoadjuvant chemotherapy was administered mainly to patients with colorectal cancers (both synchronous and metachronous metastases). Among patients with PMCA, it was predominately given for synchronous tumours. Only 3 patients with DPAM received neoadjuvant systemic chemotherapy. Hyperthermic intraperitoneal injection of oxaliplatin was used for 60 patients and mitomycin C was used for 72 patients (Table 2). Intra-abdominal use of mitomycin C was predominant among patients with appendiceal tumours and oxaliplatin use was more common among patients with colorectal cancers (p = 0.05). Among patients with colorectal cancers, intra-abdominal oxaliplatin use was significantly higher in those with synchronous tumours (p = 0.019).
Characteristics of patients according to type of intra-abdominal chemotherapy
Survival outcomes
Appendiceal tumours
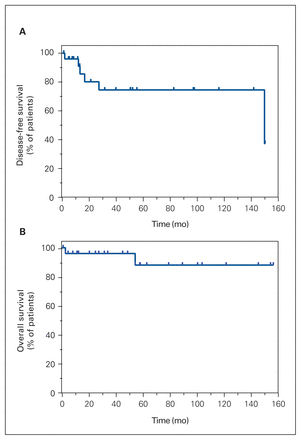
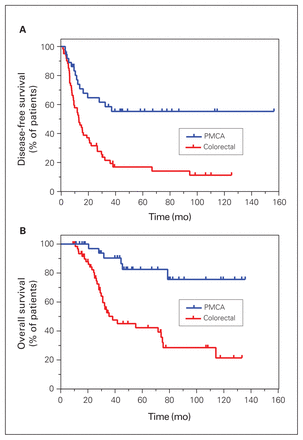
The median DFS for patients with DPAM was 149.8 months; median OS was not reached (Figure 2). Among patients with PMCA, median DFS and OS were not reached (Figure 3). Estimated 10-year survival rates of DPAM and PMCA were 89% and 75%, respectively.
Kaplan—Meier curves for (A) disease-free survival and (B) overall survival among patients with disseminated peritoneal adenomucinosis who underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Kaplan—Meier curves for (A) disease-free survival and (B) overall survival among patients with colorectal and peritoneal mucinous carcinomatosis (PMCA) who underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
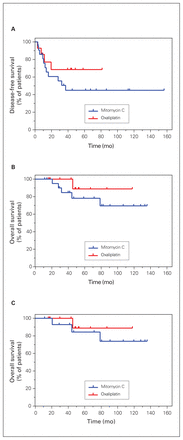
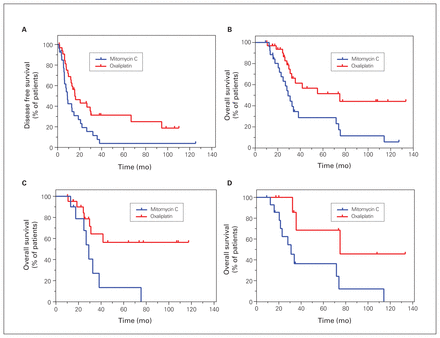
In patients with PMCA, intraperitoneal HIPEC with oxaliplatin showed a trend toward better DFS and OS, although this was not statistically significant, when compared with intra-abdominal use of mitomycin C (p = 0.28 for DFS and p = 0.33 for OS). The median DFS for the mitomycin C group was 37.1 months; it was not possible to calculate it for the oxaliplatin group. The median OS was not reached for either chemotherapy group (p = 0.55; Figure 4A and 4B). The same trend toward better OS was also seen in patients with synchronous PMCA tumours (p = 0.33; Figure 4C); the median OS was not reached for these patients.
Kaplan–Meier curves for (A) disease-free survival and (B) overall survival among patients with PMCA, according to the type of intraperitoneal chemotherapy used. (C) Kaplan–Meier curves for overall survival among patients with synchronous peritoneal mucinous carcinomatosis, according to the type of intraperitoneal chemotherapy used.
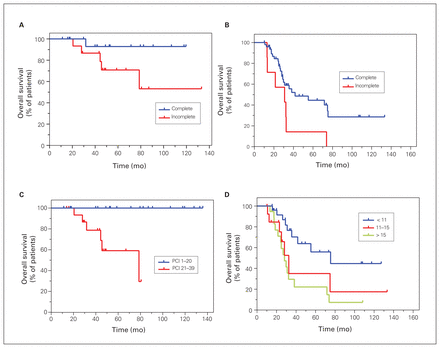
Completeness of cytoreduction and PCI score were found to be prognostic factors and predictive of better survival. Although the median OS was not reached in patients with PMCA who underwent complete (CC-0) or incomplete resection (CC-1 and CC-2), complete resection was associated with better survival (p = 0.08; Figure 5A). Lower PCI scores were also associated with better survival. The median OS was not reached in patients with PMCA and PCI scores of 1–20 whereas the median OS was 78.7 months for those with PCI scores of 21–39 (p < 0.001; Figure 5C).
Kaplan–Meier curves of overall survival according to completeness of cytoreduction for patients with (A) PMCA and (B) colorectal tumours, and according to PCI score for patients with (C) PCMA and (D) colorectal tumours. PCI = peritoneal carcinomatosis index; PMCA = peritoneal mucinous carcinomatosis.
Colorectal tumours
Patients with colorectal tumours with PC had a worse prognosis than those with PMCA; the median DFS and OS were 13 and 38.3 months, respectively (p < 0.001 for both comparisons) (Figure 3). The estimated 5-year survival rate was 42%.
Intraperitoneal HIPEC with oxaliplatin was associated with better survival. The median DFS for patients who received oxaliplatin was 15.7 months, compared with 9 months for those who received mitomycin C (p = 0.017; Figure 6A). Median OS was 74.9 and 29.1 months for patients who received oxaliplatin and mitomycin C, respectively (p = 0.006; Figure 6B). The significant improvement in OS with intra-abdominal use of oxaliplatin was still observed in both synchronous and metachronous colorectal tumours. The median OS was not reached in patients with synchronous tumours who received HIPEC with oxaliplatin; it was 29.1 months in these patients who received mitomycin C (p = 0.015). OS was 74.9 months for patients with metachronous tumours who received oxaliplatin and 31 months for those who received mitomycin C (p = 0.022) (Figure 6C and 6D).
Kaplan–Meier curves of (A) disease-free survival and (B) overall survival among patients with colorectal cancer, and Kaplan– Meier curves of overall survival in patients with (A) synchronous tumour and (D) metachronous tumours, according to the type of intraperitoneal chemotherapy used.
Complete cytoreduction and lower PCI score were associated with better survival in patients with colorectal cancers. The median OS was 41.7 and 31.0 months for patients who underwent complete and incomplete cytoreductive surgery, respectively (p = 0.02; Figure 5B). Patients who had a PCI score less than 11 had better survival than those with a score of 11–15 or more than 15 (75.4 mo v. 32.6 mo v. 28.1 mo; p = 0.002) (Figure 5D). Although this finding was not statistically significant, left-sided colorectal tumours were associated with better survival than right-sided tumours; median OS was 41.7 versus 35.6 months (p = 0.94; Figure 7).
Kaplan–Meier curve of overall survival among patients with colorectal tumours, according to the side of the primary tumour. LT = left-sided tumour; RT = right-sided tumour.
Discussion
Peritoneal carcinomatosis was once considered a terminal disease and treated with only palliative systemic therapies. It carried a poor prognosis with a maximum reported median OS of 15 months.5 Later, PC was considered a regional form of metastasis that warranted local aggressive therapies in selected patients. Surgical resection, defined initially by Sugarbaker as cytoreductive surgery, and HIPEC were hence further developed.7
CRS is a long procedure, with potential multiorgan resections and bowel anastomosis. It is associated with a high risk of intraoperative blood loss and morbidities. In our centre, mean intraoperative blood loss was merely 548 mL, and blood transfusion was needed in only 25.5% of patients. Our patients had a mean hospital stay of 16.75 days, and the mean CCI score was low. The morbidity profile of our patients was comparable to that of patients at other centres.22–24 Our centre is considered a reference centre in the province of Quebec, Canada, where the services of a large, dedicated, experienced surgical team, together with early intervention by all needed specialists, led to a low complication rate. Initially, all patients were admitted for observation to the intensive care unit (ICU), but lately patients have been admitted to the ICU only as indicated.
To our knowledge, there have been no prospective randomized controlled trials studying the benefit of neoadjuvant chemotherapy in PC of both colorectal and appendiceal tumours. However, studies have shown that perioperative chemotherapy for resectable liver metastasis of colorectal origin did not improve overall survival.25 In our cohort, liver metastases were mainly capsular lesions. Systemic chemotherapy was offered to reduce tumour size, which could improve outcomes and allow for easier and more complete resection, organ preservation and less morbidity. Neoadjuvant chemotherapy was offered to only 54.6% of our patients, who had mainly colorectal and PMCA cancers. Preoperative systemic chemotherapy was offered to a few patients with DPAM, because the waiting time to surgery is long in Quebec and according to our local experience some patients did have a response. A retrospective study of 34 patients with PMCA showed a short-term survival benefit in patients with complete response only.26 Thus, systemic neoadjuvant chemotherapy is offered for better surgical feasibility, not for survival benefit. Future randomized controlled trials are warranted to clarify the indications of neoadjuvant therapy in this setting.
In our study, HIPEC with oxaliplatin was associated with better DFS and OS in patients with colorectal cancer when compared with the use of mitomycin C, but the difference was not statistically significant in patients with PMCA. Leung and colleagues, in a retrospective analysis of 201 patients, showed that use of intraperitoneal oxaliplatin for PC of colorectal adenocarcinomas was associated with better survival outcomes, particularly in patients with non–signet cell tumours.27 On the other hand, the American Society of Peritoneal Surface Malignancies (ASPSM) demonstrated that use of mitomycin C was associated with slightly better OS (a finding that was not statistically significant), but in subgroup analysis, the use of mitomycin C improved survival in patients with low burden disease who had complete resection.28 A propensity score matched analysis confirmed that hyperthermic oxaliplatin use during HIPEC did not increase morbidities or mortalities.29,30
Thus, the choice of intraperitoneal chemotherapy may vary according to several factors, including histology sub-types (grade of tumour and presence or absence of signet cells), disease burden and patients’ response to exposure to oxaliplatin in previous systemic therapy. It should be noted that in the PRODIGE 7 study, a randomized controlled trial of 265 patients with PC secondary to colorectal tumours, HIPEC with oxaliplatin did not improve survival when compared with CRS only. This trial is the only randomized controlled one present to date in the literature.9 In our centre, intraperitoneal mitomycin C in appendiceal tumours is usually used to allow longer exposure of the peritoneum to the chemotherapy, as prolonged peritoneal oxaliplatin use is associated with increased risk of peritoneal bleeding. On the other hand, mitomycin C is used for HIPEC for patients with PC of colorectal origin whose disease is resistant or has progressed after systemic oxaliplatin therapy. Thus, with these strategies, mitomycin C is used for patients whose disease is already assocaited with a worse prognosis, which can explain the survival results we obtained.
In our cohort, patients with appendiceal tumours had prolonged survival with CRS and HIPEC, with an estimated 10-year survival rate of 89% and 75% for DPAM and PMCA subtypes, respectively. These results were slightly higher than those reported by Chua and colleagues in a retrospective study, where patients with appendiceal mucinous neoplasms and peritoneal involvement had a median OS of 16.3 years, with 10- and 15-year survival rates of 63% and 59%, respectively.31 On the other hand, patients with colorectal cancer in our cohort had a median OS of 38.3 months and a 5-year survival rate of 42%. This was similar to the results of most published studies, in which median OS ranged between 32 and 41 months.32–34
Completeness of cytoreduction and PCI score are considered important prognostic factors, as patients with CC-0 and a low PCI score had the highest survival rate for both appendiceal and colorectal tumours. In patients with colorectal tumours, complete cytoreduction was associated with better survival than incomplete cytoreduction, with a median OS of 41.7 months, which is similar to the survival results in the PRODIGE 7 study.9,10 In that trial, all patients received intra-abdominal oxaliplatin infusion and only patients with a PCI score less than 25 were included. In contrast, in our study, mitomycin C was used in cases of oxaliplatin resistance. In the PRODIGE 7 study, patients with PCI scores less than 11 had the highest survival, but subgroup analysis showed that patients with PCI scores of 11–15 benefited the most from HIPEC. The correlation between PCI and survival was also obvious in our study, but we lacked a comparative group of patients treated with CRS without HIPEC. In another retrospective study, Elias and colleagues showed that patients with PCI scores less than 19 had better survival than those with PCI scores higher than 19.35
Similar results were observed in appendiceal tumours in our study, as patients with PCI scores less than 20 had much better survival than those with PCI scores of 20 and more. Jimenez and colleagues cited PCI as a prognostic factor in a retrospective study of 387 patients, in which patients with PCI scores less than 20 had 5-year OS of 100% and 60% in DPAM and PMCA, respectively; these were significantly different than the results for patients with PCI greater than 20.36
Sidedness of the primary tumour in colorectal cancer plays an important role in prognosis in some series. Right-sided tumours are associated with worse survival than left-sided tumours.37,38
We performed resection of hepatic and subcapsular lesions in 23% of our patients. The presence of liver metastases was initially considered a contraindication to CRS and HIPEC. Later, a curative approach was considered in patients with a limited number of liver metastases.39 In a French retrospective analysis of 43 patients who underwent CRS and HIPEC for peritoneal carcinomatosis of colorectal origin, there was no difference in survival between patients with or without liver metastases.40 A recent meta-analysis of retrospective studies showed that patients with isolated PC experienced better survival than patients with both PC and liver metastases undergoing CRS and HIPEC, but the survival of patients in the latter group was still better than that of patients who underwent systemic chemotherapy alone.2,41,42 Therefore, a curative approach should still be considered in selected patients with both hepatic and peritoneal metastases.
Limitations
Our study has several limitations. It was a retrospective study with a limited number of patients and thus it is not ideal to draw conclusions for daily clinical practice. Some of the long-term complications may not have been fully reported, as some patients were followed in different outside clinics after discharge and thus we sometimes lacked detailed follow-up data. Moreover, our surgeons may have created a highly selected patient population by performing CRS and HIPEC only on well, fit patients. In addition, the study lacked a comparison group of patients who underwent CRS without HIPEC, and thus it is not possible to fully determine the indications for and benefits of adding HIPEC to CRS. Further prospective trials are warranted to establish these indications.
Conclusion
CRS-HIPEC is an acceptable, safe therapeutic treatment option for patients with PC. It is considered by many as the standard of care in patients with appendiceal tumours, and it is gaining more popularity in peritoneal carcinomatosis secondary to colorectal tumours and other abdominal malignancies. To date, there exists no high-level evidence that clearly shows a benefit of the addition of HIPEC to CRS. Yet criteria for selecting the patients that will benefit the most from this procedure are variable, and the criteria for selecting patients for the addition of HIPEC are not well established. Further prospective randomized clinical trials are warranted for this population.
Footnotes
Competing interests: F. Mercier has received grants and personal fees from Ipsen and personal fees from Advanced Accelerator Applications.
Contributors: R. Younan and M. Tehfé designed the study. R. Nassabein, R. Loungarath, F. Mercier, F. Dagbert, F. Aubin and J.-P. Ayoub acquired the data, which R. Nassabein, R. Younan and F. Dagbert analyzed. R. Nassabein and R. Younan wrote the article, which R. Younan, R. Loungarath, F. Mercier, F. Dagbert, F. Aubin, J.-P. Ayoub and M. Tehfé critically revised. All authors gave final approval of the version to be published.
- Accepted April 27, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/