Abstract
The apprentice model has traditionally been the primary method of teaching cardiac surgery trainees. Limitations of this model include insufficient time to learn all necessary skills, minimal exposure to rare cases and to complex repair techniques, small number of patients in small centres, high cost and absence of objective measures of feedback. In recent years, simulation-based training (SBT) has been used in order to address the gaps left by the apprentice model. We performed a systematic review of PubMed and Embase for articles investigating the use of SBT in teaching surgical valve techniques published in 2022 or earlier in order to summarize the current literature regarding the use of SBT for trainees learning surgical valve repair and replacement techniques. We compiled data on the impact of SBT on time to completion of tasks, proportion of trainees who committed technical errors, skills scores and theoretical knowledge. Studies in which outcomes were evaluated showed significant improvement in these measures after participation in SBT. Simulation-based training has been shown to improve the surgical skills of trainees in a rela-tively short period. As hands-on experience in the field of cardiac surgery is invaluable and often difficult to reproduce effectively, it is likely that a combination of hands-on training and SBT will be adopted moving forward to provide optimal exposure for surgical trainees.
Valvular heart disease is a relatively common condition affecting an estimated 2.5% of the population in developed nations.1–4 The mitral valve (MV) and aortic valve (AV) are the 2 most commonly diseased valves.2,4 Severe valvular heart disease is not amenable to pharmacotherapy, and, thus, the only definitive treatment is surgical repair or replacement of the diseased valve.
Heart valve repair and replacement are complex procedures requiring years of training to achieve proficiency. Surgical trainees have traditionally learned these technical skills in the operating room through the apprentice model, which entails a surgical resident’s being taught by a single mentor at a time.5,6
Although hands-on experience is irreplaceable and has a record of proven success, several limitations have been identified with the apprentice model.7,8 These limitations include insufficient time to learn all necessary skills, minimal exposure to rare and complex cases, 3-dimensional (3D) anatomy of valvular components, complex techniques, absence of objective measures of feedback, and high cost owing to the time and resources required for adequate training.5,6,9,10 Furthermore, minimally invasive valve repair techniques such as hemisternotomy, thoracotomy and catheter-based approaches are increasingly common and present unique challenges and increased surgical complexity.11–16
Surgical simulators have gained popularity in recent years as a potential solution to address the limitations of the apprentice model. Herein, we describe the current devices simulating valve repair and replacement techniques described in the literature, and their impact on trainee learning.
Methods
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, with aid from previously published descriptions of the PRISMA guidelines, when performing this systematic review.17,18 Two authors (R.E. and N.M.F.) reviewed PubMed and Embase for articles published from Jan. 1, 2000, to May 15, 2022 detailing the use of simulation-based training (SBT) for heart valve surgery. The search terms, used individually or in combination, were “simulation,” “simulation based training,” “heart valve,” “aortic valve,” “mitral valve,” “tricuspid valve,” “pulmonary valve,” “cardiac surgery,” “training” and “residents.” Exclusion criteria included SBT for surgical procedures outside of heart valve surgery, descriptions of devices without corresponding data showing the efficacy of their use, and focus on physicians other than trainees. Articles that were felt to meet the inclusion criteria were reviewed. The reviewers also examined the reference lists of reviewed studies to identify other potentially relevant articles. Disputes regarding the inclusion or exclusion of articles were resolved through discussion and consensus between the reviewers.
Use of simulators
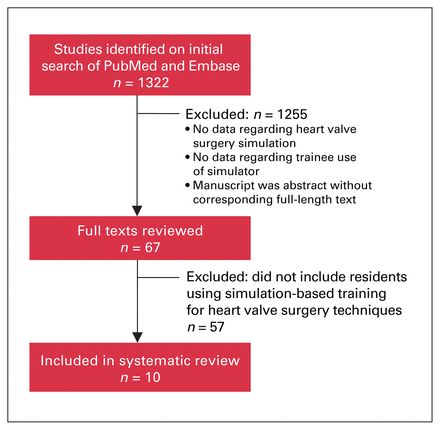
A total of 1322 articles were screened, 67 full texts were reviewed, and 10 articles were included in this review (Figure 1).
Flow diagram showing selection of studies for systematic review.
Surgical simulators can generally be divided into low-and high-fidelity devices. Low-fidelity simulators are made with readily available and cost-effective materials. With high-fidelity simulators, the simulator replicates actual anatomy. Low-fidelity simulators are advantageous as their production is simple and cost-effective; however, they are made from common materials and often do not provide a realistic look or feel. In contrast, high-fidelity simulators provide a realistic look and feel. Their main limitations are cost and accessibility, with many simulators being prohibitively expensive for widespread adoption into surgical programs.
Aortic valve simulators
Aortic valve conditions are most commonly corrected through valve replacement with a bioprosthetic or mechanical valve. The most common approaches used today are the sternotomy, hemisternotomy and transcatheter approaches, although the prevalence of thoracotomy has increased in recent years.13 In addition to native valve excision and AV replacement (AVR), the surgical procedure involves sternotomy, initiation of cardiopulmonary bypass and adequate closure of the sternotomy, all of which are complex techniques individually. In this section, we focus on models simulating AVR.
Rocha e Silva and colleagues19 described a cost-effective low-fidelity simulator produced from materials found at household supply stores, including a transparent box, plastic sewer piping, elastics, paper clips and a wooden shelf. The sewer pipes represent the aortic annulus, and a connecting piece represents the valve. A silicone mould is placed around the connecting piece. Sutures are passed through the silicone mould, representing the sutures placed in the prosthetic valve. Although the model is inexpensive, at less than US$20 (about Can$27), the materials are not realistic in feel or look. Said20 described an aortic root simulator produced from readily available materials such as silk tape, rubber gloves, tubing and plastic cups. The model allows for simulation of AV repair, AVR, aortic root replacement and remodelling techniques.
A middle-fidelity simulator described by Hossien9 includes a stable base made from a circular tin. The aortic root is made by shaping silicone over a clay mould. This produces a reusable model of the aortic root and valve with a realistic shape, allowing for AV repair and AVR. In an effort to increase the realistic feel of simulators, more recent AV models have begun incorporating patient-specific anatomy through the creation of models based on real patient cardiac imaging.21
Several high-fidelity simulators have been produced with the use of animal or human tissue and realistic procedures.13,15,22–25 Fann and colleagues22 described their AV model in which a porcine heart is placed in a container simulating the operating room exposure for AVR. Bouma and colleagues15 described their high-fidelity model used for both open and transcatheter AV repair or AVR. Their model is composed of a preserved human cadaver inside a simulated operating room. A median sternotomy is performed, and a beating heart model is prepared. After the procedure, an endoscopic view can be used to assess the quality of the repair, as an intra-aortic balloon pump allows for realistic opening and closing of the valves. Brandão and colleagues26 constructed a high-fidelity simulation model for AVR using bovine hearts situated in a synthetic box (Gbs – Simuladores Medicos) to reproduce anatomic position and surgical perspective. Donated authentic valve prostheses are used in the simulation sessions. The model described by Sharma and colleagues27 consists of porcine hearts suspended by skewers in 2-L buckets on ironing boards. Expired or obsolete AV prostheses, along with suture ring holders that are donated or made from recycled cardboard, are used to permit realistic valve replacement. The total cost of the first simulation (30 trainees enrolled) was about US$800 (about Can$1070), and subsequent sessions were free of cost, supported by local tissue donation.
Mitral valve simulators
Numerous factors increase complexity in MV surgery, including asymmetric and 3D annulus, chordae and papillary muscles, and various conditions described with the Carpentier classification. Furthermore, there are various exposure techniques, including sternotomy and thoracotomy, and several repair options, such as MV replacement (MVR), leaflet resection, neochord implantation and annuloplasty repairs.11,12,28–30 As such, considerable effort has been directed toward developing a realistic MV simulator for the various surgical approaches and scenarios.
Rocha e Silva and colleagues19 described their low-fidelity MV simulator, similar to their aortic simulator, in which a bent piece of tubing simulates access to the MV. Greenhouse and colleagues28 described their low-fidelity MV training simulator produced with materials found in any hardware store, including polyvinyl chloride and hot glue. The total cost of the model was about US$40 (about Can$54). Hossien29 described a low-fidelity MV repair (MVr) model produced with a cardboard box, drain pipe and sponge. This model allows for simulation of MVr, leaflet resection, sliding plasty, neochord implantation and annuloplasty ring repair with the use of paper clips wrapped in surgical tape mimicking annuloplasty rings. Another low-fidelity model for MV surgery, described by Verberkmoes and Verberkmoes-Broeder,31 comprises a baby bottle, latex dental dam and polyvinyl chloride adaptor. Types I, II and IIIa valve dysfunction can be simulated with this model.
Yamada and colleagues25 described their 3D MV model created with the use of data from computed tomography scans. They created a mould and filled it with polyvinyl alcohol, producing a solid model of the heart with realistic internal anatomy. They created thoracic cage models to allow for a realistic approach to the MV and to enable practice of other cardiac surgery techniques. Ginty and colleagues32 used echocardiography, modelling software and 3D printing with silicone to create patient-specific MV models for MVr for preoperative planning; the principles are transferrable for the creation of simulators for training. A heart phantom simulator (True Phantom Solutions) using realistic fluid dynamics was used in order to assess the simulated repair of the valve. Englehardt and colleagues11 also used patient-specific models created through 3D printing with a silicone base, allowing for simulation of annuloplasty, neochordae implantation and leaflet resection.
Jebran and colleagues12 identified several additional difficulties with learning minimally invasive MVr, including making the small incision and using the unique instruments required to reach into the incision. As opportunities to learn these skills are limited, those authors created a simulator to teach minimally invasive MV skills. The simulator comprised an aluminum conduit simulating the incision, allowing for simulation MVr or MVR through both thoracotomy and endoscopic approaches. Premyodhin and colleagues33 created a 3D-printed polyvinyl alcohol MV model to simulate a robotic approach to repair. They collected images of MVs using transesophageal echocardiography, and compiled the images to create the model. Sardari Nia and colleagues30 described a 2-day minimally invasive endoscopic MV training course. The course includes a theoretical portion, in which the principles of MVr are taught. General and MV-specific skills are practised with high-fidelity simulators.
Leopaldi and colleagues34 described a beating heart MVr simulator (LifeTec Group) that allows for practice with the use of beating heart transcatheter MVr and MVR devices. The model operates in real time and enables echocardiographic imaging, videoscopic vision and the development of hemodynamic changes. The biosimulator is composed of a pressurized left ventricle and a pulsatile fluid (saline) dynamic system that allows for the simulation of blood flow while enabling videoscopic imaging.
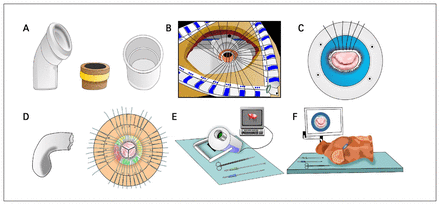
Valdis and colleagues35 described the results of an SBT program teaching students the techniques of robotic MV annuloplasty. Students with fewer than 10 hours of experience with the da Vinci Surgical System (Intuitive Surgical) were shown an instructional video and then asked to place 3 sutures through a porcine MV and an annuloplasty band. The students were then randomly allocated to 4 groups: wet laboratory, dry laboratory, virtual reality simulation or a control group. The wet laboratory group repeated the same initial tasks, the dry laboratory group learned the specific required skills through camera manipulation and peg transfers, and the virtual reality group performed 9 exercises designed to enable the trainee to acquire robotic cardiac surgical skills. The control group received no additional training on the robot. Illustrations representing these various types of simulators are included in Figure 2.
Illustrations of simulators for teaching heart valve surgery. (A) Low-fidelity simulators made up of a bent plumbing pipe and straight plumbing pipe representing the mitral and aortic valve views, respectively, and a valve model made up of a piece of pipe wrapped in a rubber band representing a sewing ring. (B) Middle-fidelity simulator modelling aortic valve replacement with a mechanical valve through a simulated sternotomy. (C) Middle-fidelity simulator modelling mitral valve repair with an annuloplasty ring in the process of having sutures tied. (D) Middle-fidelity simulator modelling the aorta and aortic root, as well as a top–down view of aortic valve replacement with sutures in place. (E) High-fidelity simulator modelling minimally invasive mitral valve repair. (F) High-fidelity simulator with a mannequin modelling minimally invasive mitral valve repair.
Joyce and colleagues36 used a 3-pronged approach in their MVr simulation program. Residents observed instructional videos, practised on a plastic MV model, and were evaluated on a porcine heart model. The plastic MV model was a silicone-based, pliable cylinder with a simulated MV annulus. The high-fidelity simulation consisted of porcine hearts situated at anatomic depth with preset ideal exposure of the MV annulus and Sorin AnnuloFlo or Sorin Annulo-Flex annuloplasty rings (Sorin Group USA). Videos of the operation captured by 2 peripheral cameras and a head-mounted camera were used to assess resident performance.
Tavlasoglu and colleagues37 described a high-fidelity simulation of MVr that permitted assessment of coaptation depth and mitral regurgitation after repair. A bovine heart model permitting pressurization of the left ventricle was used. A study table was employed to immobilize the bovine heart, which allowed “home-alone” surgical practice. Residents watched a series of 9 instructional videos before performing simulated MVr over the course of 3 months.
High-fidelity MVR simulations described by Brandão and colleagues26 and Sharma and colleagues27 are equivalent to their AVR simulations described in the previous section, with the exception that Brandão and colleagues26 used porcine hearts as opposed to bovine hearts.
Outcomes
In this section we review outcomes and changes in skill after simulator training. Study characteristics are summarized in Table 1, and the study outcomes are summarized in Table 2.
Characteristics of studies included in systematic review
Summary of outcomes after simulation-based training
Aortic valve simulators
In the study by Feins and colleagues,5 27 first-year cardiothoracic surgery residents were recruited to participate in a curriculum investigating the utility of SBT. The residents were enrolled in a 39-session curriculum that used SBT in component-based task training and skill repetition for teaching various cardiac surgery techniques. Early sessions focused on individual tasks, and later sessions progressed to simulating complete procedures. Likert scores were used to evaluate skill improvement, with a score of 5 constituting a perfect score. Residents showed improvement from their first session with the AVR assessment tool to their final session (average Likert score 3.89, 95% confidence interval [CI] 3.57–4.22 v. 4.61, 95% CI 4.43–4.79).
Sharma and colleagues27 enrolled 45 trainees (6 residents, 17 students, 8 nurses, and 14 registrars) in a 3-week low-cost, high-fidelity workshop focusing on AVR, MVR and coronary artery bypass surgery. The authors reported solely subjective outcomes in the form of self-assessment scores across 2 domains — knowledge and practical abilities. On completion of the workshop, all 4 groups reported a significant improvement in mean scores for anatomy (2.6, 95% CI 2.3–2.8 v. 3.4, 95% CI 3.1–3.7), imaging (1.9, 95% CI 1.7–2.2 v. 2.7, 95% CI 2.4–3.0) and theoretical knowledge (1.7, 95% CI 1.4–1.9 v. 3.4, 95% CI 3.1–3.7), as well as description of procedure steps (1.8, 95% CI 1.4–2.1 v. 3.3, 95% CI 3.0–3.6), performing steps (1.4, 95% CI 1.1–1.6 v. 2.9, 95% CI 2.7–3.2) and performing the operation (1.2, 95% CI 1.0–1.5 v. 2.9, 95% CI 2.6–3.1).
Brandão and colleagues26 described the results of a 5-week high-fidelity training program on AVR, MVR and coronary artery bypass surgery for second- and third-year cardiovascular surgery residents. After the simulation program, the residents received mean performance ratings on a 5-point Likert score of 4.79, 4.72, 4.47, 4.34 and 4.20 for anatomy knowledge, valve excision, stitches in the annulus, prosthesis implantation and time, respectively. Residents were not objectively evaluated before commencement of the simulation sessions, and, consequently, no quantitative analysis of improvement was performed.
Mitral valve simulators
Greenhouse and colleagues28 evaluated the effect of training level on success with the use of their model by comparing outcomes between general and cardiothoracic surgery residents and attending cardiothoracic surgeons. Times to completion of annular and sewing ring suture placement, needle angle, accuracy, suture placement and tying of the final knots were compared. There were significant differences between groups for all scores measured, with scores increasing with training level. Composite scores on a scale from 1 to 100 were provided. The scores achieved were 32.9 (standard deviation [SD] 11.4), 65.1 (SD 11.5) and 88.3 (SD 7.8) for general surgery residents, cardiothoracic surgery residents and attending surgeons, respectively (p < 0.001).
Englehardt and colleagues11 enrolled 5 expert surgeons and 7 surgical residents in a study evaluating the duration of individual knotting between the first and final knots of the same simulation. The duration of knotting improved from an initial range of 16.1–30.5 seconds to a final range of 6.6–15.7 seconds.
Jebran and colleagues12 used their model to teach 10 residents and 10 students minimally invasive MV techniques with 6 training sessions over 4 weeks. Evaluations included skills scores and time to completion. Students’ mean time to completion improved from an initial 54 (SD 5) minutes to a final 35 (SD 6) minutes (p < 0.05), and residents’ mean time improved from an initial 47 (SD 6) minutes to a final 31 (SD 6) minutes (p < 0.05). The mean skills score for students was initially 131 (SD 21), compared to 208 (SD 19) after training. (p < 0.05). The residents showed a similar improvement, from 149 (SD 21) to 237 (SD 15) (p < 0.05).
Sardari Nia and colleagues30 recruited 103 participants for a 2-day endoscopic MV surgery training course. The first day consisted of theoretical content, with a focus on basic endoscopic skills. During the second day, hands-on training focused on endoscopic mitral annuloplasty. After the course, there was a significant decrease in mean operative speed, from 87 (SD 26) seconds to 42 (SD 19) seconds (p < 0.001). There was also a significant improvement in accuracy with the posterior leaflet sutures improving from an initial 56% to 100% (p < 0.001) and the anterior leaflet sutures from 43% to 99% (p < 0.001). All participants underwent a standardized question assessment. Participants gained significant knowledge during the course, with the mean scores improving from 58% before training to 67% after training (p < 0.001).
In the study by Valdis and colleagues,35 40 surgical trainees completed a standardized robotic dissection of the internal thoracic artery and placed 3 sutures of an MV annuloplasty in porcine models. Skills were evaluated with the Global Evaluative Assessment of Robotic Skills. The authors calculated time-based scores by subtracting the time taken from 720. Participants in the wet laboratory, dry laboratory and control groups showed significant improvements in their scores, whereas participants in the virtual reality group did not. The mean final time-based scores were 602.2 (SD 11.4), 523.5 (SD 48.9), 580.4 (SD 14.4) and 463.8 (SD 86.4) for the wet laboratory, dry laboratory, virtual reality and control groups, respectively.
The high-fidelity 3-week MVr simulation study by Joyce and colleagues36 involved 11 first-, second- and third-year cardiothoracic surgery residents who had no previous clinical experience performing MV surgery. A porcine model of MV annuloplasty in combination with instructional video was used. The authors assessed skill acquisition with a modified version of the Objective Structured Assessment of Technical Skills. There was a significant improvement in feedback scores on all 11 points of the assessment. Furthermore, mean time to completion was significantly reduced after the program (31 [SD 9] min v. 25 [SD 6] min) (p = 0.03).
Tavlasoglu and colleagues37 enrolled cardiovascular surgery residents in an MVr simulation program. Ten residents (5 junior and 5 senior) underwent 3 months of training with a bovine heart model and were assessed monthly on 2 primary outcomes: coaptation depth and regurgitation score. Coaptation depth improved significantly over time in both groups (p < 0.001). Similarly, the mean regurgitation score improved each month for the junior residents (p < 0.02) and improved from the first to the second month and from the first to the third month for the senior residents (p < 0.02).
Discussion
Numerous studies have been published detailing the creation and use of surgical simulators; however, the majority of authors have focused on descriptions of simulators, with few performing objective assessments of the utility of their models. Authors who have measured the performance of their simulators have found promising results. Significant improvement in surgical trainees’ time to completion of tasks, number of mistakes, skills scores and theoretical knowledge after participation in SBT has been reported.5,11,12,26–28,30,35–37 The use of SBT in training for heart valve surgery is relatively limited compared to other surgical fields, such as coronary revascularization.38 As such, further investigation into the use of SBT for teaching valve repair and replacement techniques is warranted to identify the optimal use of SBT for training surgical residents.
Several limitations to the apprentice model of training surgical residents have been identified, including insufficient time to learn all the necessary skills, minimal exposure to rare and complex cases, 3D anatomy of valvular components, complex repair techniques and absence of objective feedback measures.5,6,9,10 Simulation-based training has been shown to be able to address these limitations. The apprentice model has traditionally restricted learners to the assistant’s position, especially during complex cases and early in training, owing to the limited ability for early learners to perform these complex procedures safely with patients. Although senior residents have shown acceptable outcomes when acting as the primary surgeon, surgeons with increased experience have also been reported to have results superior to those of surgeons with less experience.7,8,39,40 Limitations of the apprentice model are not easily remedied. It has been proposed that SBT may be able to fill the gaps left by the apprentice model in order to provide optimal training for surgical residents. Simulations will likely play a larger role in surgeon training in the future to fill these gaps. This additional experience will be invaluable, give that cardiac surgical procedures are becoming increasingly complicated, with an increase in the prevalence and efficacy of complex, minimally invasive and hybrid surgical techniques.41
Simulation-based training allows learners to take the position of the surgeon and provides the opportunity to perform complex or novel procedures early in their surgical training and before extensive exposure to a specific procedure without conferring direct risk to patients. This allows trainees to gain exposure to techniques years earlier than they otherwise would have been able to. Residents who are exposed to SBT and have the opportunity to apply skills and techniques earlier may have increased confidence and reduced risk of error when they receive the opportunity to apply those skills in the operating room. In addition, there are rare conditions and surgical procedures that are not seen at every centre. In these cases, SBT via surgical simulator or case-based simulation may be an option to enable residents to gain these skills and experience, even if such cases do not present spontaneously to their centre.
As surgical procedures and patients’ conditions become increasingly complex, surgical trainees will continue to experience increasing pressure to learn an ever-growing body of knowledge and necessary surgical skills. For these reasons, we feel SBT should be implemented early in surgical training and continued throughout residency, as it can be useful even for cases that residents have not had ample exposure to in the operating room. At our centre, SBT is used in the curriculum for resident training. Beginning early in their training, residents are given the opportunity to practise surgical techniques during simulation sessions. In regular simulation sessions, they practise on high-fidelity simulators or porcine models, under the supervision of surgical and anesthetic preceptors. Residents can try new surgical devices and techniques, or practise learned techniques in a controlled environment. During weekly half-day sessions, residents are exposed to low-fidelity or case-based simulation. Finally, residents are exposed to dedicated surgical simulators and are encouraged to engage in surgical simulation, which often includes practising techniques on low-fidelity simulators, during free time.
Simulation-based training does have several inherent shortcomings, with the main limitations being cost and lack of exposure to realistic scenarios. Although cost-effective simulators have been described, such as those of Greenhouse and colleagues19 and Rocha e Silva and colleagues,28 they are produced with materials that do not have a realistic feel or look. High-fidelity simulators with increased realism are costly, often being prohibitively expensive when considering widespread use with a large number of surgical trainees. Furthermore, even with high-fidelity simulators, aspects of real-world cases and patients are difficult to replicate. These include individual variations between patients, the incidence of unexpected complications, incidental findings intraoperatively, communication among the members of a large surgical team, and other interpersonal skills required for a successful surgical career.
Although the data presented in this review show the efficacy of SBT in training surgical residents, this training method cannot perfectly replicate the operating room experience and feel during surgery. A balance must be found with SBT when integrated into a training program so that operating room time is not compromised for residents. In programs in which there is dedicated academic or teaching time, high-fidelity SBT may be used to provide high-quality learning without compromising operating room time. In addition, low-fidelity simulators and case-based simulation are cost-effective options and can be used by all residents in a training program for practice during times when they are otherwise not engaged in the practice of surgical techniques. Furthermore, SBT must be integrated with care. Exposure to SBT without adequate instruction or supervision may result in suboptimal learning and even reinforcement of incorrect technique. During early exposure to SBT or specific techniques, instruction from experienced surgeons is essential to ensure proper technique is being performed. When trainees are comfortable with a technique, they may transition to individual practice, which will allow for increased practice time with SBT while ensuring proper technique is being used.
The ideal use of SBT will vary from program to program. Some programs may find the highest yield in establishing SBT for junior trainees and continuing it throughout their residency training. This will allow for the early establishment of essential surgical skills and the continual improvement throughout their training. Residency training programs should look to provide hands-on instruction from experienced surgeons when trainees are first learning a new technique and allow them to practise on their own once they have developed proper technique and comfort with the simulator. This will allow residents to establish a strong surgical base and provide the opportunity for ample exposure to SBT. Finally, residents should be exposed to a variety of SBT tools, if possible. The use of high-fidelity simulators provides a high-yield experience but is not feasible at all times. Therefore, the use of low-fidelity simulators for residents to practise on their own time combined with dedicated sessions of SBT with high-fidelity simulators may provide a suitable balance for optimal learning. The integration of case-based simulation into SBT programs is of great importance in providing exposure to rare cases and an additional form of teaching. Each program, as well as individual residents, may require variation in their SBT program, which necessitates communication and feedback among training programs and their trainees in order to establish the optimal curriculum.
In the coming years, we will likely see continual introduction of SBT into cardiac and other surgical training programs, with optimal SBT use being identified for each specialty or program. The most efficient and successful methods of training residents in surgical valve replacement in the future will probably involve a combination of hands-on learning with a supervisor and SBT.
Limitations
There are limitations to our study. Relatively few authors measured performance after training with heart valve surgery simulators. In addition, not all studies included objective measures of performance, which limits interpretation of the results of individual studies and the number of studies included in this review. Finally, among studies in which objective measures of feedback were used, the measured variables and metrics employed varied from study to study, which limited the ability to directly compare studies or pool data.
Conclusion
Simulation-based training has been shown to improve the surgical skills of trainees in a relatively short period. It has been proposed as an adjunct to traditional surgical training in order to fill the gaps left by the apprentice model. As hands-on experience in the field of cardiac surgery is invaluable and often difficult to reproduce effectively, it is likely that a combination of hands-on training and SBT will be adopted moving forward to provide optimal exposure for surgical trainees.
Acknowledgement
The authors thank Dawne Colwell, medical graphics designer, for creating the figures.
Footnotes
Presented as an abstract at the virtual Canadian Conference for the Advancement of Surgical Education, Sept. 30–Oct. 1, 2021
Competing interests: None declared.
Contributors: All authors designed the study. R. EL-Andari, J. Kang and N. Fialka acquired the data, which all authors analyzed. R. EL-Andari, S. Bozso and J. Kang wrote the manuscript, which S. Bozso, N. Fialka, C. Adams and J. Nagendran critically revised. All authors gave final approval of the article to be published.
Funding: This work was supported by the University Hospital Foundation, University of Alberta.
- Accepted July 18, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/