Abstract
Background: Current measures to prevent spinal surgical site infection (SSI) lack compliance and lead to antimicrobial resistance. We aimed to examine the effectiveness of bundled preoperative intranasal photodynamic disinfection therapy (nPDT) and chlorhexidine gluconate (CHG) body wipes in the prophylaxis of spine SSIs in adults, as well as determine our institutional savings attributable to the use of this strategy and identify adverse events reported with nPDT–CHG.
Methods: We performed a 14-year prospective observational interrupted time-series study in adult (age > 18 yr) patients undergoing emergent or elective spine surgery with 3 time-specific cohorts: before rollout of our institution’s nPDT–CHG program (2006–2010), during rollout (2011–2014) and after rollout (2015–2019). We used unadjusted bivariate analysis to test for temporal changes across patient and surgical variables, and segmented regression to estimate the effect of nPDT–CHG on the annual SSI incidence rates per period. We used 2 models to estimate the cost of nPDT–CHG to prevent 1 additional SSI per year and the annual cumulative cost savings through SSI prevention.
Results: Over the study period, 13 493 patients (mean 964 per year) underwent elective or emergent spine surgery. From 2006 to 2019, the mean age, mean Charlson Comorbidity Index (CCI) score and mean Spine Surgical Invasiveness Index (SSII) score increased from 48.4 to 58.1 years, from 1.7 to 2.6, and from 15.4 to 20.5, respectively (p < 0.001). Unadjusted analysis confirmed a significant decrease in the annual number (74.6 to 26.8) and incidence (7.98% to 2.67%) of SSIs with nPDT–CHG (p < 0.001). After adjustment for mean age, mean CCI score and mean SSII score, segmented regression showed an absolute reduction in the annual SSI incidence rate of 3.36% per year (p < 0.001). The estimated annual cost to prevent 1 additional SSI per year was about $1350–$1650, and the estimated annual cumulative cost savings were $2 484 856–$2 495 016. No adverse events were reported with nPDT–CHG.
Conclusion: Preoperative nPDT–CHG administration is an effective prophylactic strategy for spinal SSIs, with significant cost savings. Given its rapid action, minimal risk of antimicrobial resistance, broad-spectrum activity and high compliance rate, preoperative nPDT–CHG decolonization should be the standard of care for all patients undergoing emergent or elective spine surgery.
Surgical site infections (SSIs) are devastating postoperative complications associated with substantial morbidity, prolonged length of stay (LOS) and increased health care–related costs.1 The rate of SSIs after spine surgery is estimated at 1%–16%,2,3 with 67.1% of affected patients requiring further surgery.4 Multiple patient and surgical risk factors are associated with SSIs after spine surgery.2–14 Increased age, greater comorbidity burden, implantation of spinal hardware, greater surgical invasiveness, distant site infection and positive methicillin-resistant Staphylococcus aureus (MRSA) cultures are the most consistent.2–14 Strategies for preventing SSIs represent an important opportunity to improve patient care and reduce health care–related costs.
Initiatives for reducing the risk of SSIs have been at the forefront of surgical practice and medical literature.15–17 Of these, bundled SSI prophylaxis interventions have gained particular interest. Bagga and colleagues18 reported that the SSI incidence rate was reduced from 3.4% to 1.2% with preoperative chlorhexidine washing and standardized postoperative wound care in a cohort of more than 9000 patients. Featherall and colleagues19 reported a decrease in the SSI rate from 4.1% to 2.0% with a preoperative bundle that included screening and decolonization for S. aureus with nasal mupirocin administration and self-preparation bath with chlorhexidine gluconate (CHG). However, the gold-standard SSI prophylaxis strategy remains unknown, given institutional variability in SSI rates, allocation of resources and cost constraints.
Nasal administration of mupirocin is the decolonization technique for SSI prophylaxis most frequently reported. It has demonstrated efficacy as a single prophylactic measure,20 and Yamada and colleagues21 reported a reduction in SSI rates of 3.1% when mupirocin was part of a bundled approach in patients undergoing spine surgery. The accessibility, cost-effectiveness and favourable study outcomes with nasal administration of mupirocin have made it a relatively popular decolonization technique. However, its effectiveness depends on patient compliance and microbiologic susceptibility.22 The latter is particularly concerning, given the increasing rate of mupirocin-resistant organisms.23,24 Given these limitations, there is a need for an alternative nasal decolonization technique, ideally one without the potential for antimicrobial resistance.
Intranasal photodynamic disinfection therapy (nPDT) is a novel nonantibiotic broad-spectrum antimicrobial technology in which light energy is used to activate a photoactive agent to kill microbial cells in the anterior nares.22 This strategy has been shown to eliminate intranasal carriage of MRSA and methicillin-susceptible S. aureus, with sustained decolonization for up to 5 days.25 In 2011, as part of a year-long pilot study at our institution, Bryce and colleagues26 showed that bundled nPDT–CHG was most effective in the spinal surgery population, with an 18-fold reduction in the odds of developing an SSI due to S. aureus. The combination of nPDT and CHG decolonization has the potential advantage of broad-spectrum antimicrobial efficacy and a lower risk of antimicrobial resistance.26
Since 2006, we have been prospectively collecting SSI data before, during and after the implementation of nPDT–CHG at our institution. Our primary objective in the present study was to examine the effect of nPDT–CHG on the yearly cumulative incidence rate of SSIs in adult patients undergoing emergent or elective spine surgery. Our secondary objectives were to determine our institutional savings attributable to the use of this strategy and identify adverse events reported with nPDT–CHG.
Methods
Setting and design
Vancouver General Hospital is a level 1 trauma and quaternary spine referral centre in British Columbia, Canada, serving about 5 million inhabitants. We are the regional centre for complex spine care, including complex adult spinal deformity, trauma and oncology.
This prospective observational interrupted time-series study contains data from Jan. 1, 2006, to Dec. 31, 2019, as part of a quality-improvement initiative for preventing SSIs. Our study population consists of all adult patients (age > 18 yr) undergoing elective or emergent spine surgery for each calendar year.
Preoperative surgical site infection prophylaxis bundle
The preoperative SSI prophylaxis bundle consists of decolonization treatment with CHG-impregnated wipes (Sage Products) to wash the surgical site, axillae and groin within 24 hours preceding surgery, and nPDT (Ondine Biomedical) administered in the preoperative holding area. Patients undergoing elective surgery are given information on the decolonization program in the preadmission clinic or surgeon’s office. Patients undergoing emergency surgery are given the information by the admitting surgical service. The nPDT component of the decolonization treatment is administrated by trained nurses. This includes applying a photosensitive dye (0.1% methylene blue solution) to the anterior nares for 30 seconds, followed by 2 cycles of 2-minute illumination with a nonthermal red light wavelength of 665 nm.26
Rollout of preoperative surgical site infection prophylaxis bundle
In 2011, a preoperative SSI prophylaxis quality-improvement program was introduced as a pilot study at our institution.26 From Sept. 1, 2011, to Aug. 31, 2012, patients undergoing an elective cardiac, orthopedic, spinal, vascular, thoracic or neurosurgical procedure were recruited to examine the effectiveness of the nPDT–CHG treatment as described above.26
After the success of the pilot study, nPDT–CHG treatment was implemented into full-time practice in 2 phases. Phase I of the rollout (2012–2013) included all elective and emergency surgical procedures performed from 0700 to 1600, Monday to Friday. In phase II of the rollout (2013–2014), the program was gradually expanded to include all elective and emergency surgical procedures performed at any time, any day of the week. By Jan. 1, 2015, all patients undergoing elective or emergent procedures received nPDT–CHG in the preoperative holding area before transfer to the operating room.
Patient and surgical data
We prospectively collected standardized patient and surgical data, including demographic information, Charlson Comorbidity Index (CCI) score, case diagnostic category (deformity, oncology, trauma or degenerative), neurologic status and Spine Surgical Invasiveness Index (SSII) score. The CCI is a validated measure that assesses 19 comorbidities to produce a weighted score predictive of short- and long-term health outcomes.27 We evaluated surgical invasiveness using the SSII.11 We categorized the SSII scores into mild (< 10), moderate (10–21) and major (> 21) invasiveness.
Outcomes of interest
Our primary outcome of interest was the cumulative change in the annual incidence rate of SSIs after the full-time implementation of preoperative nPDT–CHG, in 2015. We used the US Centers for Disease Control and Prevention National Healthcare Surveillance Network definition of SSI.28 Cases of SSI were confirmed by the attending surgeon and institutional SSI surveillance program (Infection Prevention and Control) using laboratory data, review of the surgical cases list, voluntary reporting, reports from other facilities and review of hospital readmissions with a diagnosis of infection. We collected all cases of confirmed SSI prospectively using our prospective adverse event database, the Spine Adverse Events Severity system, version 2.12,13
Our secondary outcomes of interest were adverse events specific to the administration of nPDT–CHG and the cumulative institutional savings attributable to nPDT–CHG decolonization. We defined adverse events using the Spine Adverse Events Severity system, version 2, a validated, spine-specific, physician-led prospective adverse event database for 14 specific intraoperative and 22 postoperative adverse events12,13 (Appendix 1, available at www.canjsurg.ca/lookup/doi/10.1503/cjs.016922/tab-related-content). Adverse events specific to the administration of nPDT–CHG are recorded under the “other” section for each patient.
Cost models
We developed 2 different cost models. The first was an estimate of the nPDT–CHG cost to prevent 1 additional SSI per year. The second was an estimate of cumulative institutional savings after implementation of the SSI prophylaxis program. We derived the base cost of nPDT–CHG per patient of $45–$55 from institutional financial reports. The base cost for treating each confirmed case of SSI in our model was $52 932. We derived this figure from the study by Barnacle and colleagues,29 in which the estimated cost for treating each confirmed case of SSI was NZD$51 434. We standardized values to 2022 Canadian cost estimates using a 2018 New Zealand dollar to Canadian dollar conversion rate of 0.8973,30 then adjusted for inflation using data from the Bank of Canada.30 We used data from New Zealand to estimate the Canadian equivalent for several reasons. Current Canadian data evaluate the economic impact of pooled medical and surgical adverse events, which dilutes the specific cost of treating spine SSIs.31,32 Health economic data across the US literature are variable, with estimates of the cost of SSI treatment per case ranging from US$16 2421 to US$93 741;33 this range reflects the competing financial interests between federal and private health care institutions and insurance programs.33,34 The 2018 cost estimate of Barnacle and colleagues29 represents the most recent health economic data specific for treating spine SSIs, without dilution costs, in a public health care model comparable to that in Canada.35,36
Model 1
We derived the cost of nPDT–CHG to prevent 1 additional SSI per year from the number needed to treat, which we calculated using the adjusted absolute percent change in the annual SSI incidence rate per year from our segmented regression analysis (intercept parameterization region A v. region C). We then multiplied the number needed to treat by the approximate cost of nPDT–CHG per patient, which yielded an average estimate for the total cost of nPDT–CHG to prevent 1 additional SSI per year.
Model 2
We determined the cumulative institutional savings by calculating the differences in the annual SSI cost per year between the prerollout period (2006–2011) and the postrollout period (2015–2019), adjusting for the yearly cost of administering nPDT–CHG from 2015 onward. We calculated the prerollout annual SSI cost per year by multiplying the annual number of confirmed SSIs before rollout by the estimated cost of treating an SSI (PreCT = annual number of SSIs Prerollout × $52 932). We calculated the postrollout annual SSI cost per year by multiplying the annual number of confirmed SSIs after rollout of the SSI prophylaxis program by the estimated cost of treating an SSI (PostCT = annual number of SSIs Postrollout × $52 932). We calculated the annual cost of administering nPDT–CHG per year after program rollout by multiplying the annual number of surgical cases by the cost of administering nPDT–CHG per patient (CTPDT = annual surgical cases after rollout per year × $45–$55). Finally, we calculated the annual cumulative institutional savings with adjustment for the cost of administrating nPDT–CHG using the formula InstCS = PreCT − PostCT − CTPDT.
Statistical analysis
We used descriptive statistics to summarize the patient, surgical and outcome variables of our study population using means, standard deviations (SDs), proportions or rates, as appropriate. We used unadjusted bivariate analysis using the Pearson correlation coefficient to test for temporal changes across individual patient and surgical variables. We used an unadjusted independent 2-sample t test to test the difference in the mean number and incidence of SSIs per year between the pre- and postrollout periods.
We developed a segmented regression model to determine the effect of the nPDT–CHG program on the yearly SSI incidence rate, adjusting for independent predictors of SSI.37 The SSI incidence rate was recorded every calendar year from 2006 to 2019. We developed the segmented regression model with 2 joinpoints: 2011 (last year of the prerollout period) and 2014 (last year of the rollout period). The model was constrained to meet at each joinpoint, while allowing for different year slopes to be fitted within regions A (prerollout), B (rollout) and C (postrollout). The model included the covariates mean age, mean CCI score and mean SSII score.
We parameterized the model in several ways to numerically determine the effect of the nPDT–CHG program on the yearly SSI incidence rate. First, we parameterized the model to calculate the slope (β) representing the adjusted annual SSI incidence rate per year for each region. We then parameterized the model to compare the difference in slopes between regions, representing the adjusted change in the annual SSI incidence rate per year between the prerollout, rollout and postrollout periods. Finally, we parameterized the model by comparing the difference in intercepts (α) between regions, representing the adjusted absolute percent change in the annual SSI incidence rate per year between the prerollout, rollout and postrollout periods. We confirmed model equivalence numerically between the various parameterizations by checking the other effects in the models. A 95% confidence interval (CI) was provided with all effect estimates from the segmented regression analysis. We performed the analyses using SAS version 9.4 (SAS Institute).
Ethics approval
This study was approved by the Vancouver Coastal Health Regional Ethics Committee and the University of British Columbia Clinical Research Ethics Board (H10-03441) as a quality-improvement project. The study was conducted following the principles of the Helsinki Declaration. The requirement for informed consent was waived because of the anonymous nature of the data.
Results
Patient demographic characteristics and case distribution
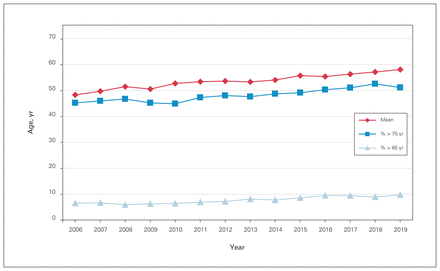
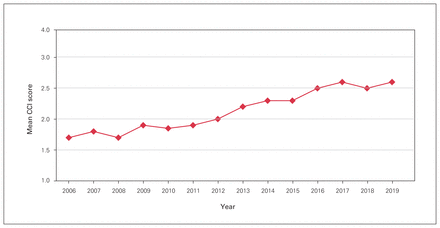
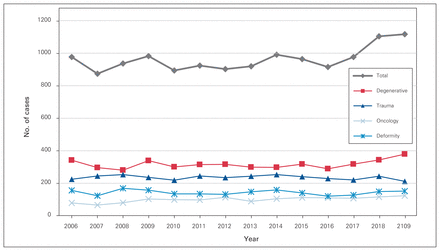
Over the study period, 13 493 patients underwent elective or emergent spine surgery at our institution, with 4670 cases before program rollout, 3741 during the rollout period and 5082 after program rollout. There was a mean of 964 (SD 73) surgical cases per calendar year (Table 1). From 2006 to 2019, the mean age increased by a decade, from 48.4 to 58.1 years (r = 0.98, p < 0.001). The proportion of patients older than 75 years of age and older than 85 years of age increased from 45.3% to 51.2% and from 6.5% to 9.8%, respectively (r = 0.93, p < 0.001) (Figure 1). The mean CCI score and mean SSII score increased from 1.7 to 2.6 (r = 0.97, p < 0.001) and from 15.4 to 20.5 (r = 0.88, p < 0.001), respectively (Figure 2). The proportion of SSII scores greater than 21 increased from 52.5% to 61.0% (r = 0.82), and the proportion of SSII scores less than 10 decreased from 14.0% to 8.0% (r = −0.90) (p < 0.001). The proportion of oncology cases increased from 8.0% to 11.0% (r = 0.66, p = 0.011), and the proportion of deformity and trauma cases decreased from 16.0% to 13.5% (r = −0.64, p = 0.01) and from 23.0% to 19.0% (r = −0.587, p = 0.03), respectively (Table 2 and Figure 3). There were no changes in the proportion of degenerative surgical cases during the study period (r = −0.33, p = 0.2).
Patient age distribution over the study period.
Mean Charlson Comorbidity Index (CCI) score over the study period.
Distribution of cases by diagnostic category over the study period.
Patient demographic characteristics by year
Surgical demographic characteristics by year
Pearson correlation analysis identified significant associations between mean age and mean CCI score (r = 0.91, p < 0.001), mean age and proportion of oncology cases (r = 0.69, p = 0.001), and proportion of oncology cases and mean SSII score (r = 0.75, p = 0.002). Overall, over the study period, there was a statistically significant increase in mean patient age of a decade; in patient comorbidity burden, by almost 50%; in surgical invasiveness, by 20%; and in the proportion of surgical oncology cases, by 40%.
Cumulative surgical site infection incidence rate
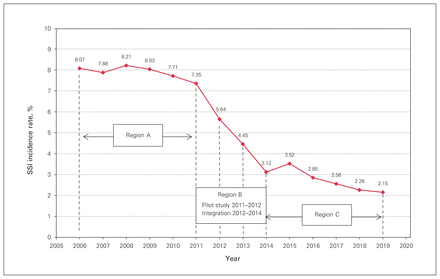
Before program rollout, the mean number of confirmed SSIs per year was 74.6 (SD 5.18), with a maximum SSI incidence rate of 8.21% and a minimum incidence rate of 7.71% (Table 3 and Figure 4, region A). During the rollout period, the mean number of confirmed SSIs per year was 47.75 (SD 15.78), with a maximum SSI incidence rate of 7.35% and a minimum incidence rate of 3.12% (Figure 4, region B). After rollout, the mean number of confirmed SSIs per year was 26.8 (SD 4.09), with a maximum SSI incidence rate of 3.52% and a minimum incidence rate of 2.15% (Figure 4, region C). Unadjusted analysis confirmed a significant reduction in the mean number and mean incidence of SSIs per year between the pre- and postrollout periods (p < 0.001).
Cumulative surgical site infection (SSI) incidence rate over the study period. Region A: before rollout of the SSI prophylaxis program; region B: program rollout; region C: after program rollout.
Incidence of surgical site infection by year
Segmented regression analysis with slope parameterization identified that, after adjustment for mean age, mean CCI score and mean SSII score, program rollout (region B) was associated with a statistically significant reduction in the annual SSI incidence rate of 1.32% (95% CI −1.95 to −0.69) per year (Table 4). Parameterization of slope differences between regions confirmed that the greatest percent change in the annual SSI incidence rate occurred only during rollout, compared to the pre- or postrollout period (p < 0.001). Intercept parameterization between the prerollout period (region A) versus the postrollout period (region C) showed that, after adjustment for mean age, mean SSII score and mean CCI score, program rollout was associated with a statistically significant absolute percent reduction in the annual SSI incidence rate of 3.36% (95% CI −4.67 to −2.04) per year. None of the adjustment variables were statistically significant except for mean SSII score, which had borderline significance (p = 0.06).
Segmented regression analysis*
Adverse events
No adverse events associated with nPDT–CHG were reported or observed during the study.
Cost of intervention and cumulative institutional savings
With model 1, after adjustment for mean age, mean CCI score and mean SSII score, the estimated annual cost associated with nPDT–CHG to prevent 1 additional spine SSI per year during program rollout was about $1350–$1650.
With model 2, the estimated annual prerollout SSI treatment cost was $3 969 900 per year, and the estimated mean postrollout treatment cost was $1 429 164. The annual cost of administering nPDT–CHG after rollout was $45 720–$55 880. Therefore, the estimated annual cumulative institutional savings attributable to the use of nPDT–CHG was $2 484 856–$2 495 016 per year.
Discussion
Our study showed that the preoperative use of nPDT–CHG was associated with a significant reduction in the annual SSI incidence rate of 5.31% per year. After adjusting for age, surgical invasiveness and comorbidity burden, we observed a significant reduction in the annual SSI incidence rate of 1.32% per year during nPDT–CHG rollout and an absolute reduction in the annual yearly SSI incidence rate of 3.36% per year. The use of nPDT–CHG also offered estimated cumulative institutional savings of $2 484 856–$2 495 016 annually. In addition, no adverse events were reported, which suggests that nPDT is a safe technology.
The cumulative reduction in the incidence of SSIs occurred during significant changes in our study population over time. Concurrent with the aging population, we observed a significant increase in patient age of a decade, a 50% increase in mean CCI score, a 20% increase in mean SSII score and a 40% increase in the proportion of oncology surgery cases performed each year. The CCI score is one of the strongest independent risk factors for SSI after spine surgery, with a reported hazard ratio of 2.48,38 and older age almost doubles the odds of developing an SSI.39,40 Moreover, we observed an increase in overall mean SSII scores and proportion of SSII scores greater than 21, which may reflect the yearly increase in oncology cases. A significant dose–response relation exists between SSII score and infection, with a score greater than 21 increasing the relative risk of SSI by 3.15 times.9 Patients undergoing spine surgery for oncologic conditions also experience a greater incidence of SSI, with reported rates as high as 4.4%41 and 9.5%.42 Despite significant increases in all these risk factors in our population, bundled preoperative nPDT–CHG remained an effective intervention.
There are limited studies investigating the effect of nPDT–CHG. Our results are consistent with a meta-analysis of the use of similar preoperative bundled SSI prevention strategies.43 Yamada and colleagues21 observed a reduction in the SSI rate from 3.8% to 0.7% and an absolute risk reduction of 84% with nasally administered mupirocin and CHG skin decolonization. Similarly, Schweizer and colleagues44 reported a risk reduction of 42% with a bundled SSI prevention strategy in complex SSI due to S. aureus after cardiac, hip or knee surgery. We observed that, after full implementation of the nPDT–CHG program, the slope representing the annual SSI incidence rate plateaued, with a loss of statistical significance. The plateau observed in the postrollout period likely reflects the maximal preventive effect of nPDT–CHG in patients undergoing spinal surgery. The insignificant difference in adjusted slope between the pre- and postrollout periods also suggests that, after full implementation of the program, there was a return to the baseline variability in yearly SSI incidence. This residual baseline variability, saturated by the maximal preventive effect of nPDT–CHG, represents the baseline SSI risk attributable to the medical and surgical complexity of our patient population devoid of colonization. These findings further validate the effectiveness of nPDT–CHG in reducing the risk of SSI associated with nasal and skin colonization in a high-risk population.
Since full implementation of the nPDT–CHG program, we have implemented other changes in surgical practice at our institution to prevent SSIs, such as intra-wound administration of vancomycin powder, wound drains, negative pressure wound dressings and insertion of silver-coated Foley catheters. These factors were not accounted for in the present study, as they occurred only after rollout and their use is highly variable depending on surgeon preference, case complexity and patient factors. Furthermore, our analysis identified that the only significant change in yearly SSI incidence rate occurred during nPDT–CHG rollout, which validates the effectiveness of this SSI prophylaxis strategy. This is in keeping with the finding of Bryce and colleagues26 that nPDT–CHG decolonization resulted in greater reduction in SSI incidence in patients undergoing elective cardiac, orthopedic, spinal, vascular, thoracic or neurosurgical procedures than in a historical control group and a concurrent control group, reducing the odds of SSI due to S. aureus by 18 fold.
The effectiveness of nasally administered mupirocin depends on patient compliance and antibiotic susceptibility.23,26 Nicolas and colleagues45 reported that a therapeutic mupirocin concentration was found in the nasal secretions of only 22 of 41 patients who reported good compliance with nasal self-administration of mupirocin. Hetem and colleagues23 reported a rate of mupirocin resistance in coagulase-negative Staphylococcus of 96% after nasal decolonization with mupirocin. In comparison, preoperative nPDT has been found to have excellent patient compliance (94%), ease of use without interrupting nursing workflow and an average administration time of 10 minutes.26 Bundled nPDT and CHG skin decolonization also offers the theoretical advantage of broad-spectrum antimicrobial efficacy, rapid action and low risk of development of antimicrobial resistance.26
Our cost analysis identified that the estimated cost of nPDT–CHG to prevent 1 additional SSI per year was $1350–$1650 per year. Our estimated annual institutional cumulative savings attributable to nPDT–CHG use was $2 484 856–$2 495 016 per year, adjusted for the ongoing annual cost of administering nPDT–CHG to all patients after program rollout. Interestingly, our cumulative institutional savings were substantially greater than those previously reported. Stambough and colleagues46 estimated a net savings of US$717 205.59 within 25 months after implementing a decolonization program that reduced the incidence of SSI from 0.8% to 0.2% in patients undergoing elective total hip and knee replacement. Rennert-May and colleagues47 reported a cost saving of $153 per person and 16 SSIs avoided annually with nasally administered mupirocin and CHG skin decolonization in more than 8000 hip or knee replacement procedures. The institutional savings observed in our study reflects the effectiveness of nPDT–CHG in a high-risk surgical population and the avoided substantial costs of treating spine SSIs.29 Accordingly, bundled nPDT–CHG may be a financially viable long-term solution, given its affordability and clinical effectiveness.
We observed no additional adverse events specific to nPDT–CHG use in our prospective adverse event database. Bryce and colleagues26 reported 7 cases of transient pharyngeal irritation, likely related to trickling of the methylene blue into the back of the throat, but no other complications. Two of the 7 patients were referred to otorhinolaryngology for nasopharyngoscopy, and no tissue reaction was observed on examination. Those authors did not report any cases of altered taste or smell related to the nasal treatment.
Limitations
Our findings must be interpreted within the context of the study design. Its prospective nature, large sample, long follow-up period and direct comparison of pre- versus post-intervention cohorts, together with the fact that rigorous SSI surveillance continued unaltered after full-time implementation of the nPDT–CHG program, lend strength to our findings. In addition, the interrupted time-series design and use of segmented regression analysis are validated methodologies for evaluating the effectiveness of largescale health interventions implemented at specific times and for testing causal hypotheses about the intervention.48–50
Although no additional interventions were introduced during nPDT–CHG rollout, our study did not account for variabilities and changes in other practice standards. The increase in SSI awareness and prevention has introduced changes in nursing care, medical education and generalized ward management. Although these factors cannot be accounted for, it remains that the significant reduction in SSIs occurred only during nPDT–CHG rollout.
Surveillance data are constrained by the lack of individualized data. Accordingly, we could not account for the number of patients who received nPDT–CHG from 2011 to 2014. However, as nPDT–CHG was a defined intervention with specific independent time points, the difference in SSI prevention cost between the pre- and postrollout periods provides a reasonably accurate estimate of our institutional savings attributable to the use of nPDT–CHG. Comparing defined time points also eliminates any assumptions made during nPDT–CHG rollout that may skew the cost estimates. Likewise, by incorporating the ongoing annual cost of administering nPDT–CHG to all patients after rollout, our model reflects a more accurate estimate of our institutional savings. Last, the scope of SSI prevention has a far greater impact than cost savings alone: it also substantially reduces the physical, emotional and medical burden on patients and families.
Finally, our study did not investigate whether there were differences or changes in the etiologic organism or antimicrobial resistance patterns in patients who developed an SSI. These are clinically important questions to address, as they provide insight into whether preoperative nPDT–CHG is associated with changes in the medical and surgical management of patients who develop an SSI postoperatively. Future studies are needed to determine whether preoperative nPDT–CHG decolonization is associated with changes in etiologic SSI organism(s), antimicrobial resistance patterns, length of antibiotic treatment or differences in surgical management51 such as the number of surgical washouts and/or hardware revisions.
Conclusion
Preoperative bundled nPDT–CHG is a clinically effective strategy for reducing the incidence of SSIs after emergent or elective spine surgery. It is an affordable intervention and is associated with significant institutional savings for every SSI prevented in this high-risk population. Given its rapid action, minimal risk of antimicrobial resistance, broad-spectrum activity and high compliance rate, preoperative bundled nPDT–CHG decolonization should be the standard of care for all patients undergoing emergent or elective spine surgery.
Acknowledgements
This work would not have been possible without the dedication and hard work of the staff of the Vancouver Spine Research Group, and the ongoing support of the Bob and Trish Saunders Spine Research Fund through the VGH & UBC Hospital Foundation.
Footnotes
Competing interests: None declared.
Contributors: J. Street supervised the project. E. Moskven, D. Banaszek, E. Sayre, A. Gara, E. Bryce, T. Wong and J. Street designed the study. J. Street, E. Bryce, T. Wong, D. Banaszek and E. Moskven acquired the data, which E. Moskven and E. Sayre analyzed. E. Moskven, D. Banaszek, T. Ailon, R. Charest-Morin, N. Dea, M. Dvorak, C. Fisher, B. Kwon, S. Paquette and J. Street wrote the article, which E. Moskven, D. Banaszek, T. Ailon, R. Charest-Morin, N. Dea, M. Dvorak, C. Fisher, B. Kwon, S. Paquette and J. Street reviewed. All authors approved the final version to be published.
- Accepted September 7, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/